Abstract
Purpose
Ultrasonography allows the direct observation of the diaphragm. Its thickness variation measured in the zone of apposition has been previously used to diagnose diaphragm paralysis. We assessed the feasibility and accuracy of this method to assess diaphragmatic function and its contribution to respiratory workload in critically ill patients under non-invasive ventilation.
Methods
This was a preliminary physiological study in the intensive care unit of a university hospital. Twelve patients requiring planned non-invasive ventilation after extubation were studied while spontaneously breathing and during non-invasive ventilation at three levels of pressure support (5, 10 and 15 cmH2O). Diaphragm thickness was measured in the zone of apposition during tidal ventilation and the thickening fraction (TF) was calculated as (thickness at inspiration − thickness at expiration)/thickness at expiration. Diaphragmatic pressure–time product per breath (PTPdi) was measured from oesophageal and gastric pressure recordings.
Results
PTPdi and TF both decreased as the level of pressure support increased. A significant correlation was found between PTPdi and TF (ρ = 0.74, p < 0.001). The overall reproducibility of TF assessment was good but the coefficient of repeatability reached 18 % for inter-observer reproducibility.
Conclusions
Ultrasonographic assessment of the diaphragm TF is a non-invasive method that may prove useful in evaluating diaphragmatic function and its contribution to respiratory workload in intensive care unit patients.
Similar content being viewed by others
Introduction
Work of breathing is a central physiologic parameter in the assessment of a critically ill patient’s respiratory function [1]. The diaphragm is the main respiratory muscle and plays a central role in the pathophysiology of respiratory failure [2]. Evidence for ventilator-induced diaphragmatic dysfunction is now well established in animal models [3–5] and in critically ill patients [6–8]. On the other hand, optimal setting of the ventilator should theoretically target a clinically acceptable level of work of breathing. Most assisted modes of ventilation have been studied regarding their ability to reduce the work of breathing, especially during weaning from mechanical ventilation or non-invasive ventilation (NIV) [9, 10]. Several methods have been used in the clinical research setting in critically ill patients to assess diaphragmatic function, including diaphragm electromyography, measurement of pleural (or oesophageal) and gastric pressures and derived variables (work of breathing) [11]. This highlights the need for simple and accurate methods to assess diaphragmatic performance in the intensive care unit (ICU) setting. Ultrasonographic-based determination of diaphragm excursions may help identify patients with diaphragmatic dysfunction during weaning from mechanical ventilation [12–14]. Ultrasonography also allows direct visualization of the diaphragm’s thickness in its zone of apposition. Measurement of the thickening of this muscle segment was described back in 1989 [15] and has been used to assess diaphragm contraction in healthy subjects or to diagnose diaphragm paralysis [16]. We hypothesized that this measurement could reflect the magnitude of diaphragmatic work and could help clinicians to optimize ventilator settings. Because pressure support ventilation is known to reduce the work of breathing, the relationship between TF and the level of pressure support was of special interest. However, the accuracy and feasibility of this method have not been evaluated in ICU patients to date. The purpose of our study was to determine whether the assessment of diaphragm thickening in its zone of apposition could be feasible, accurate and reliable in critically ill patients and to assess its usefulness as an index of respiratory workload under NIV.
Methods
Patient selection
Patients intubated for 48 h or more, who tolerated a 1 h spontaneous breathing (SB) trial after recovery from their acute disease [9], were extubated and considered eligible for the study. Patients at high risk of respiratory distress requiring early NIV after extubation were enrolled if they had at least two of the following risk factors for respiratory failure after extubation: age older than 65 years, chronic obstructive pulmonary disease (COPD) or other type of chronic respiratory failure, heart failure as a cause for intubation, and an Acute Physiology and Chronic Health Evaluation [17] (APACHE) II score greater than 12 on the day of extubation [18, 19]. Exclusion criteria were as follows: tracheostomy, age younger than 18 years, head trauma or surgery, recent gastric or oesophageal surgery, active upper gastrointestinal bleeding, excessive amount of respiratory secretions, poor cooperation, and decision to limit life-supporting treatments in the ICU. All included patients gave their informed consent to participate in the study, which was approved by the appropriate ethics committee (Comité de Protection des Personnes, Ile de France IX, approval number 05-025).
NIV
NIV was applied via a standard ICU ventilator (the type depending on availability), with an oronasal mask, in pressure support (PS) mode, as per the standard procedure in our unit. Baseline measurements were performed during SB. Recordings were then obtained during three NIV periods (with 5, 10 and 15 cmH2O of PS level), in random order. Positive end-expiratory pressure (PEEP) was maintained at 5 cmH2O and the inspired oxygen fraction was kept constant throughout the three NIV periods. Each of these three periods, as well as the SB period, lasted about 15 min. Pressure and flow data were recorded during the last 5 min of each condition after 5–10 min with a stable breathing pattern. The ultrasound examination was performed concomitantly.
Ultrasonography
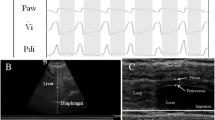
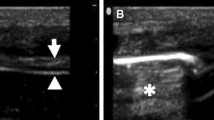
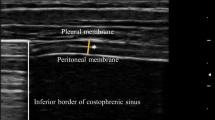
The patients were studied in the semi-recumbent position throughout the study. Ultrasonography was performed by two intensivists both experienced in ultrasonography, using an Envisor system (Philips Ultrasound, Bothell, WA) equipped with a high resolution 12 MHz ultrasound linear probe. Using the signal from the ventilator, airway pressure was displayed on the screen of the Echo-Doppler machine during NIV, ensuring accurate determination of the tracings relative to the respiratory cycle. Ultrasound recordings of diaphragm thickness were performed as previously reported [15]. Briefly, the diaphragm was located by placing the transducer in the intercostal space above the right 10th rib in the midaxillary or anteroaxillary line and directing the ultrasound beam perpendicular to the diaphragm (Fig. 1a). The zone of apposition was assessed at 0.5–2 cm below the costophrenic sinus. The inferior border of the costophrenic sinus was identified at end-inspiration as the zone of transition from the artefactual representation of normal lung (the lung sliding) to the visualization of diaphragm and liver. The diaphragm thickness was recorded in time motion (TM) mode. The sweep speed was adjusted as slow as possible to obtain a minimum of three cycles on the same image. The diaphragm was outlined by the two clear bright parallel lines of the pleural and peritoneal membranes (Fig. 1b and Online Videos). Several images were recorded and images were deemed invalid if the two clear bright parallel lines of the pleural and peritoneal membranes were not plainly identified at each moment of the respiratory cycle. Ultrasonographic recordings were stored on compact disks, and a subsequent computer-assisted quantitative analysis was performed by a trained investigator who was unaware of the ventilatory condition. The measurements included diaphragm thickness at end-expiration (T EE) and at end-inspiration (T EI). When airway pressure could not be displayed on the screen of the Echo-Doppler machine to match the ultrasound tracings to the respiratory cycle (during SB), T EE was measured just before the thickening start and T EI was measured at maximal thickening. Measurements were averaged out of three or more consecutive breaths on the last valid image recorded at the end of each period. The thickening fraction (TF) was calculated as (T EI − T EE)/T EE and expressed as a percentage (Fig. 1c).
Probe placement to explore the diaphragm in the zone of apposition (a), with the ultrasonographic view of the normal diaphragm in the zone of apposition (b) and illustration of the measurement of diaphragm thickness at end-inspiration and end-expiration in TM mode (c). T EI thickness at end-inspiration, T EE thickness at end-expiration
Flow and pressure measurements
Flow was measured using a Fleisch N°2 pneumotachograph (Fleisch, Lausanne, Switzerland) connected to a differential (±2 cmH2O) pressure transducer (MP45, Validyne, Northridge, CA) and placed between the face mask and the ventilator Y connector. Airway opening pressure was measured between the ventilator circuit and the pneumotachograph using a pressure transducer (MP45, ±100 cmH2O). Oesophageal (P es) and gastric pressures (P ga) were measured using a double-balloon catheter (Marquat, Boissy Saint Léger, France) as previously described with appropriate placement checked and artefacts eliminated (see Supplementary Material for details) [20, 21]. Transdiaphragmatic pressure (P di) was obtained by electronic subtraction of the P es signal from the P ga signal over at least ten consecutive breaths selected at the end of the pressure and flow recordings. The transdiaphragmatic pressure–time product (PTPdi) per breath was obtained by measuring the area under the P di signal from the onset of its positive deflection to its return to baseline. A difference between the beginning of the negative oesophageal-pressure deflection and the zero-flow point was taken as reflecting intrinsic PEEP [22] and was corrected for any abdominal pressure activity [23].
Assessment of TF reproducibility
Ten recordings (from ten separate patients) were randomly selected from the study to assess analyser reproducibility. The same sets of recordings were analysed twice by the same ultrasonographer to assess intra-analyser reproducibility and separately by two different ultrasonographers to assess inter-analyser reproducibility. Observer reproducibility was assessed in five additional patients (not included in the study) under NIV and during SB [24]. The two ultrasonographers performed separate recordings in these patients, one of them performing them twice in each patient (30 recordings in total). Multiple recordings on the same patient were performed in a blind fashion and separated by a time interval of 5–10 min. Each ultrasonographer subsequently analysed his recordings. Repeated measurements obtained in each patient from the same ultrasonographer were compared to assess intra-observer reproducibility. Inter-observer reproducibility was assessed by comparing measurements obtained separately from the two ultrasonographers in the same patient.
Statistical analysis
Data were analysed using the SPSS Base 13.0 statistical software package (SPSS, Inc, Chicago,IL). Continuous variables are reported as median (25–75th percentiles). Friedman test and Wilcoxon paired tests (with post hoc Bonferonni correction) were used to assess differences between related variables. Correlations between TF and respiratory variables were assessed as well as those between the directional changes (absolute difference) in TF and in PTPdi. All correlations were calculated using the Spearman method and performed for the entire data set (including all four respiratory conditions of each patient) because of the limited sample size. The reproducibility is expressed by the intra-class correlation coefficient [25] and the coefficient of repeatability [26], as proposed by Bland and Altman. Intra-class correlation coefficient was determined with consistency and 95 % confidence interval. Coefficient of repeatability is calculated as the British Standards Institution repeatability coefficient (twice the standard deviation of the differences in repeated measurements) [26]. Two-tailed p values less than 0.05 were considered significant.
Results
Patients’ characteristics
Fourteen patients were screened for the study. Of these, two could not be assessed because of poor ultrasonographic image quality. Twelve patients were therefore included in the comparison study. Their clinical characteristics are listed in Table 1.
Ultrasonography and pressure measurements
Respiratory data and ultrasound measurements are reported in Table 2. As expected, the tidal volume increased with increasing PS, but the respiratory rate did not significantly change. Increasing PS was associated with decreased PTPdi and TF (Table 2; Fig. 2). TF was significantly correlated with PTPdi (ρ = 0.74, p < 0.001; Fig. 3) but not with expired tidal volume (ρ = −0.05, p = 0.76). There was also a significant correlation between TF and PTPdi expressed per minute (ρ = 0.52, p < 0.001). The directional changes in TF after a change in the PS level followed reasonably with those in PTPdi, with a correlation coefficient of 0.35 (p = 0.04).
Diaphragmatic pressure–time product (a) and thickening fraction (b) during spontaneous breathing and during non-invasive ventilation at different pressure support levels. PTP di diaphragmatic pressure–time product, TF thickening fraction, SB spontaneous breathing, PS 5 pressure support level of 5 cmH2O, PS 10 pressure support level of 10 cmH2O, PS 15 pressure support level of 15 cmH2O
Reproducibility of TF
Repeatability measurements are reported in Table 3. Intra-class correlation coefficients were all above 0.97. Coefficients of repeatability ranged around 7–8 % for intra- or inter-analyser repeatability and around 15–18 % for intra- or inter-observer repeatability.
Discussion
This preliminary study is the first to evaluate the usefulness of diaphragm thickening to assess work of breathing in ICU patients. We found a parallel decrease in PTPdi and TF during NIV with increasing PS level and there was a good correlation between the two parameters.
Diaphragmatic pressure–time product per breath (PTPdi), which is the integration of the area under the transdiaphragmatic pressure curve versus time, is a very useful tool for quantifying respiratory muscle effort in mechanically ventilated patients and assess the oxygen cost of breathing [27]. It is one of the most direct measures of patient effort, but its execution requires considerable attention, confining its use to the research setting [28–30]. We used this method as a reference standard to evaluate the accuracy of an alternative non-invasive method to assess diaphragmatic function and its contribution to respiratory workload. Ultrasonography can be used to directly image the diaphragm. Its identification depends upon bright echoes reflected from the attached parietal pleural and peritoneal membranes [31]. Ultrasonography has been previously used to monitor displacement of the diaphragm dome during respiratory manoeuvres with a 3–5 MHz transducer [14, 32–34]. It has also been used to assess the length and thickness of the zone of apposition against the rib cage, at different lung volumes in 2D mode [35] or TM mode [15, 36, 37]. Because the costal part of the diaphragm is relatively close to the skin surface, it is possible to use a 8–15 MHz transducer in this setting, which has less penetration but enhanced resolution.
Data from a necropsy study indicate that measurement of diaphragm thickness with ultrasonography in the zone of apposition is as accurate as measurements performed in vitro with a ruler [15]. These authors also showed that ultrasound measurement of diaphragm thickness changes during contraction (namely the TF), was possible using the TM mode and linearly correlated with lung volumes in spontaneously breathing healthy volunteers [15]. In our study, it was interesting to note that TF values did not correlate with expired volumes. In fact, using different levels of PS ventilation, the expired volume was determined not only by the respiratory effort, but also by the pressure provided by the ventilator. Our results therefore show that during NIV, thickening of the diaphragm reflects muscle effort and not the increase in pulmonary volume induced by ventilation. In fact, we found a parallel decrease in PTPdi and TF with increasing PS and a significant correlation between both parameters. Diaphragm thickening monitoring was recently proposed to assess for potential functional recovery from diaphragm weakness or paralysis [38]. Our study suggests that the TF could be used in the ICU setting to assess diaphragmatic function and its contribution to respiratory workload in various situations, including ventilator-induced diaphragmatic dysfunction and ICU-acquired paresis. Moreover, because the directional changes in TF after a change in the PS level followed reasonably with those in PTPdi, TF seems promising in providing relevant information on patients’ ventilatory support needs.
A common limitation of validation studies is reporting of reproducibility based solely on repeated analyses of the same recordings. We therefore assessed reproducibility based on repeated recordings in addition to repeated analyses [24]. Mathematically, the intra-class correlation coefficient is the proportion of the total variance which is due to the variation between the subjects [39]. An intra-class correlation coefficient of 1 indicates that the total variance is due solely to the variation between the subjects, there being no contribution to the total variance from variation between the observers; whereas an intra-class correlation coefficient of 0 indicates that none of the total variance is due to variation between subjects and all the total variance is attributed to variation between observers. We found an overall good repeatability of TF assessment, with intra-class correlation coefficients well above the 0.75 cut-off usually considered to indicate good agreement [40]. This finding is similar to a previous study reporting a good reproducibility of this index [15]. The coefficient of repeatability which is expressed in the measurement units is directly related to the 95 % limits of agreement and is the smallest significant difference between repeated measurements [26]. In our study, the coefficient of repeatability was high for intra-observer and inter-observer reproducibility (15–18 %). In clinical practice, an 18 % absolute change or difference in TF may therefore be required for accurate interpretation if the recordings are made and analysed by different observers or over prolonged intervals. Whether this agreement is acceptable or not depends on the range of the measurements encountered in clinical practice [26]. In our work, TF values ranged from 0 to 80 % for the clinical study. Median values of TF dropped by more than 30 % between SB and PS at the highest level of 15 cmH2O. The relatively high coefficient of repeatability of inter-observer reproducibility highlights the fact that the repeatability of recordings may be difficult to achieve in some patients. In fact, the precise localisation of the zone of apposition and achievement of a stable ultrasound tracing are not simple, especially in case of increased respiratory workload. In addition the experience of the ultrasonographer is of importance, as with other echographic tools. On the other hand, the intrinsic respiratory variability of the patient may explain part of the variations observed when assessing the repeatability of the method because measurements were separated by a time interval of 5–10 min and only few cycles were selected for the measurements. The rhythmic activity of the respiratory central pattern generators is characterized by a breath-to-breath variability, which differs from patient to patient and is influenced by the respiratory load [41]. Using only three respiratory cycles for TF measurement as previously suggested [15, 37] may be insufficient in patients with a high respiratory variability. Future studies may consider using a minimum of ten respiratory cycles to measure TF, as is the standard for PTPdi measurements. In addition, although all efforts were made to maintain the probe exactly on the same location during the entire study protocol, we can not exclude subtle displacements that may have altered the reproducibility.
Our study has some limitations. First, we only assessed the right hemi-diaphragm because its visualization is easier as compared to the left side whose imaging is often impeded by gastric and intestinal gas interposition. Second, we could not explore two patients because of poor quality images and this preliminary study has a limited sample size. Third, we studied a specific group of patients during post-extubation pressure NIV. Our results may need replication in other patients groups. In particular, whether higher PEEP levels may influence the feasibility and accuracy of the methods needs to be assessed. In fact, lung volume changes may induce a displacement of the upper border of the zone of apposition [42]. In particular, the value of this technique needs to be assessed further in COPD patients, especially as different types of hyperaeration distribution in the lung parenchyma may affect diaphragm shape and motion [43].
In conclusion, we found a parallel decrease in TF and PTPdi during NIV with increasing levels of PS. The two parameters were significantly correlated. TF may prove useful in assessing diaphragmatic function and its contribution to respiratory workload in the ICU setting, but further studies are needed to assess this technique in a greater number of patients with various diseases.
References
Roussos C, Macklem PT (1982) The respiratory muscles. N Engl J Med 307:786–797
Tobin MJ, Laghi F, Brochard L (2009) Role of the respiratory muscles in acute respiratory failure of COPD: lessons from weaning failure. J Appl Physiol 107:962–970
Anzueto A, Peters JI, Tobin MJ, de los Santos R, Seidenfeld JJ, Moore G, Cox WJ, Coalson JJ (1997) Effects of prolonged controlled mechanical ventilation on diaphragmatic function in healthy adult baboons. Crit Care Med 25:1187–1190
Le Bourdelles G, Viires N, Boczkowski J, Seta N, Pavlovic D, Aubier M (1994) Effects of mechanical ventilation on diaphragmatic contractile properties in rats. Am J Respir Crit Care Med 149:1539–1544
Sassoon CS, Caiozzo VJ, Manka A, Sieck GC (2002) Altered diaphragm contractile properties with controlled mechanical ventilation. J Appl Physiol 92:2585–2595
Hussain SN, Mofarrahi M, Sigala I, Kim HC, Vassilakopoulos T, Maltais F, Bellenis I, Chaturvedi R, Gottfried SB, Metrakos P, Danialou G, Matecki S, Jaber S, Petrof BJ, Goldberg P (2010) Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. Am J Respir Crit Care Med 182:1377–1386
Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, Bouyabrine H, Courouble P, Koechlin-Ramonatxo C, Sebbane M, Similowski T, Scheuermann V, Mebazaa A, Capdevila X, Mornet D, Mercier J, Lacampagne A, Philips A, Matecki S (2011) Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 183:364–371
Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB (2008) Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358:1327–1335
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, Welte T (2007) Weaning from mechanical ventilation. Eur Respir J 29:1033–1056
Brochard L, Mancebo J, Elliott MW (2002) Noninvasive ventilation for acute respiratory failure. Eur Respir J 19:712–721
American Thoracic Society/European Respiratory Society (2002) ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med 166:518–624
Kim SH, Na S, Choi JS, Na SH, Shin S, Koh SO (2010) An evaluation of diaphragmatic movement by M-mode sonography as a predictor of pulmonary dysfunction after upper abdominal surgery. Anesth Analg 110:1349–1354
Kim WY, Suh HJ, Hong SB, Koh Y, Lim CM (2011) Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med 39:2627–2630
Lerolle N, Guerot E, Dimassi S, Zegdi R, Faisy C, Fagon JY, Diehl JL (2009) Ultrasonographic diagnostic criterion for severe diaphragmatic dysfunction after cardiac surgery. Chest 135:401–407
Wait JL, Nahormek PA, Yost WT, Rochester DP (1989) Diaphragmatic thickness–lung volume relationship in vivo. J Appl Physiol 67:1560–1568
Gottesman E, McCool FD (1997) Ultrasound evaluation of the paralyzed diaphragm. Am J Respir Crit Care Med 155:1570–1574
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A (2006) Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med 173:164–170
Nava S, Gregoretti C, Fanfulla F, Squadrone E, Grassi M, Carlucci A, Beltrame F, Navalesi P (2005) Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med 33:2465–2470
Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J (1982) A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 126:788–791
Diehl JL, Lofaso F, Deleuze P, Similowski T, Lemaire F, Brochard L (1994) Clinically relevant diaphragmatic dysfunction after cardiac operations. J Thorac Cardiovasc Surg 107:487–498
Pepe PE, Marini JJ (1982) Occult positive end-expiratory pressure in mechanically ventilated patients with airflow obstruction: the auto-PEEP effect. Am Rev Respir Dis 126:166–170
Lessard MR, Lofaso F, Brochard L (1995) Expiratory muscle activity increases intrinsic positive end-expiratory pressure independently of dynamic hyperinflation in mechanically ventilated patients. Am J Respir Crit Care Med 151:562–569
Thorstensen A, Dalen H, Amundsen BH, Aase SA, Stoylen A (2010) Reproducibility in echocardiographic assessment of the left ventricular global and regional function, the HUNT study. Eur J Echocardiogr 11:149–159
Bland JM, Altman DG (1996) Measurement error and correlation coefficients. BMJ 313:41–42
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Field S, Kelly SM, Macklem PT (1982) The oxygen cost of breathing in patients with cardiorespiratory disease. Am Rev Respir Dis 126:9–13
Grasso S, Puntillo F, Mascia L, Ancona G, Fiore T, Bruno F, Slutsky AS, Ranieri VM (2000) Compensation for increase in respiratory workload during mechanical ventilation. Pressure-support versus proportional-assist ventilation. Am J Respir Crit Care Med 161:819–826
Jubran A, Grant BJ, Laghi F, Parthasarathy S, Tobin MJ (2005) Weaning prediction: esophageal pressure monitoring complements readiness testing. Am J Respir Crit Care Med 171:1252–1259
Sassoon CS, Light RW, Lodia R, Sieck GC, Mahutte CK (1991) Pressure-time product during continuous positive airway pressure, pressure support ventilation, and T-piece during weaning from mechanical ventilation. Am Rev Respir Dis 143:469–475
Fried AM, Cosgrove DO, Nassiri DK, McCready VR (1985) The diaphragmatic echo complex: an in vitro study. Invest Radiol 20:62–67
Boussuges A, Gole Y, Blanc P (2009) Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest 135:391–400
Harris RS, Giovannetti M, Kim BK (1983) Normal ventilatory movement of the right hemidiaphragm studied by ultrasonography and pneumotachography. Radiology 146:141–144
Houston JG, Fleet M, Cowan MD, McMillan NC (1995) Comparison of ultrasound with fluoroscopy in the assessment of suspected hemidiaphragmatic movement abnormality. Clin Radiol 50:95–98
Cohn D, Benditt JO, Eveloff S, McCool FD (1997) Diaphragm thickening during inspiration. J Appl Physiol 83:291–296
Ueki J, De Bruin PF, Pride NB (1995) In vivo assessment of diaphragm contraction by ultrasound in normal subjects. Thorax 50:1157–1161
Wait JL, Johnson RL (1997) Patterns of shortening and thickening of the human diaphragm. J Appl Physiol 83:1123–1132
Summerhill EM, El-Sameed YA, Glidden TJ, McCool FD (2008) Monitoring recovery from diaphragm paralysis with ultrasound. Chest 133:737–743
Khan KS, Chien PF (2001) Evaluation of a clinical test. I. Assessment of reliability. BJOG 108:562–567
Kramer MS, Feinstein AR (1981) Clinical biostatistics. LIV. The biostatistics of concordance. Clin Pharmacol Ther 29:111–123
Wysocki M, Cracco C, Teixeira A, Mercat A, Diehl JL, Lefort Y, Derenne JP, Similowski T (2006) Reduced breathing variability as a predictor of unsuccessful patient separation from mechanical ventilation. Crit Care Med 34:2076–2083
Loring SH (1994) Three-dimensional reconstruction of the in vivo human diaphragm shape at different lung volumes. J Appl Physiol 76:493–494
Iwasawa T, Takahashi H, Ogura T, Asakura A, Gotoh T, Shibata H, Inoue T (2011) Influence of the distribution of emphysema on diaphragmatic motion in patients with chronic obstructive pulmonary disease. Jpn J radiol 29:256–264
Acknowledgments
This study was supported by AP-HP (Assistance Publique-Hôpitaux de Paris), a non-profit publicly funded organization.
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Vivier and A. Mekontso Dessap contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (AVI 50333 kb)
Supplementary material 3 (AVI 53478 kb)
Rights and permissions
About this article
Cite this article
Vivier, E., Mekontso Dessap, A., Dimassi, S. et al. Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med 38, 796–803 (2012). https://doi.org/10.1007/s00134-012-2547-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2547-7