Abstract
Purpose
Ultrasonographic assessment of diaphragm function with patients on low levels of pressure support (PS) predicts extubation outcomes, but similar information regarding extubation success under other conditions is lacking. The purpose of this study was to determine whether ultrasound (US) measurements of the diaphragm made on various levels of PS can predict time until successful extubation.
Methods
Fifty-six intubated patients underwent ultrasound of the right hemidiaphragm during a PS wean at varying levels of pressure support (PS 5/5 cm of H2O, 10/5 cm of H2O, and 15/5 cm of H2O). The diaphragm was visualized using a 7.5–10 mHz transducer in the zone of apposition of the diaphragm to the lower rib cage. The percent change in diaphragm thickness between end-expiration and end-inspiration (∆tdi%) was calculated at each level of PS.
Results
∆tdi% >20 is a robust predictor of extubation success within 48 h of US at PS 5/5 cm of H2O and 10/5 cm of H2O (sensitivity 84.6 and 88.9 % and specificity 79.0 and 75.0 %, respectively). At PS greater than 10/5 cm of H2O, its predictive power was greatly diminished. Of nine patients who were extubated with ∆tdi% below the cutoff, 66.6 % required emergent reintubation in the next two days.
Conclusions
Diaphragm US is a valid predictor of extubation success at some but not all PS settings. Using a ∆tdi% of 20 % on PS levels up to 10/5 cm of H2O may reduce both unnecessarily prolonged intubations and prevent emergent reintubations.
Similar content being viewed by others
Introduction
Despite advances in critical care medicine, the aptitude of clinicians to correctly predict extubation outcomes is limited. Failure to extubate appropriate patients in a timely manner is associated with significant morbidity including ventilator-associated pneumonia and diaphragm muscle atrophy [1]. Prematurely discontinuing mechanical ventilation is equally as harmful and is associated with mortality rates of as high as 42 % [2]. Even with evidence-based guidelines for discontinuation of ventilatory support, reintubation rates range from 25 to 33 % [3, 4].
A clinician’s subjective ability to predict extubation is poor (sensitivity 35 %, specificity 79 %) [5], so several objective predictors of extubation have been developed to aid in clinical decision-making [6, 7]. The most widely used predictor for successful extubation is the rapid shallow breathing index (RSBI). Using a cutoff of 105 breaths/min/L, this metric has a sensitivity of 97 % and specificity of 64 % with “T-Piece” weaning trials [8]. Utilization of the RSBI is limited because many weaning trials use pressure support (PS) ventilation and the RSBI includes the contribution of accessory respiratory muscles which may mask the presence of diaphragm weakness and its inability to sustain unassisted breathing [9, 10].
Ultrasound (US) evaluation of the diaphragm has been studied as a surrogate marker for diaphragmatic function. In human studies, diaphragm thickness measured at end-inspiration is related to maximal inspiratory pressure [11] and the change in diaphragmatic thickness during inspiration strongly correlates with inspired lung volume [12]. Diaphragm US can also be used to evaluate diaphragmatic dysfunction [13] and paralysis [14]. Since the diaphragm is the major contributor to unassisted breathing, assessment of diaphragmatic function should theoretically predict extubation outcomes. To this end, US measures of diaphragmatic dome excursion have been used to predict extubation outcomes with some success [15]. This technique, however, does not directly visualize the diaphragm muscle and motion of the dome can be affected by passive displacement by the ventilator and adjacent abdominal contents [16, 17]. To overcome these limitations, US has been used to visualize the diaphragm in the zone of apposition to the rib cage. In fact, measurement and calculation of the percent change in thickness of the diaphragm over the entire respiratory cycle predict extubation outcomes at a PS of 5/5 cm H2O or on T-piece better than either the RSBI or measurements of diaphragmatic dome excursion [18, 19]. While it has been shown that US measurements of the diaphragm on T-piece or low levels PS predict extubation success, no study so far has established the validity of these measurements under other conditions. The purpose of this study is to determine whether percent change in diaphragmatic thickness as measured by US predicts extubation outcomes at various levels of pressure support.
Methods
Subjects
All adult patients (age greater than 18 years old) intubated and mechanically ventilated for greater than 24 h were prospectively recruited from the intensive care unit (ICU) at Einstein Medical Center Philadelphia, an urban tertiary care center, between July 2014 and August 2015. Patients were excluded if they had an ischemic or hemorrhagic stroke, spinal cord injury, neuromuscular disease, pregnancy, or any pathology that would obscure visualization of the right hemidiaphragm. Terminal or self-extubation within 48 h of the ultrasound was censored from the final analysis. Informed consent was obtained from the designated decision maker, either the power of attorney or legal next of kin, in accordance with the Einstein Medical Center Institutional Review Board (IRB# 4541).
Ultrasound Measurements
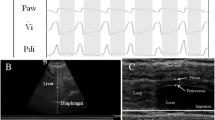
Team members included individuals formally trained in the use of ultrasound with prior clinical experience, who underwent approximately one hour of training to standardize their imaging technique and window. The diaphragm was visualized with B-Mode ultrasound using a linear 7–10 mHz probe (Sonosite M-Turbo, FujiFilm, Tokyo, Japan) at the zone of apposition located on the right mid-axillary line between the eighth and tenth ribs as previously described [12]. All subjects were evaluated with the head of the bed angled between 20° and 40° of elevation. A 15- to 30-s video clip was recorded on the ultrasound, exported, and analyzed using ImageJ (NIH, Bethesda, MD, USA). Thickness was measured from the inside edge of the diaphragmatic pleura to the inside edge of the peritoneal membrane (Fig. 1). This thickness was measured at approximately 25, 50, and 75 % along the length of the diaphragm at end-expiration (EE) and end-inspiration (EI). The average thickness was determined from these three measurements and then averaged over three respiratory cycles. The percent change in diaphragmatic thickness (∆tdi%) was calculated by (([Thickness at EI]AVG − [Thickness at EE]AVG)/[Thickness at EE]AVG) × 100. Team members analyzing the ultrasound images did not perform any of the ultrasounds and were blinded to which patients were extubated within 48 h. The intraclass correlation coefficient was 0.814.
Measurement of diaphragmatic thickness by B-Mode ultrasound. A representative ultrasound image of a patient’s diaphragm at end-expiration (a) and end-inspiration (b). The distance between the diaphragmatic pleura (arrow) and peritoneal membrane (arrow head) represents the diaphragmatic thickness. The lung (asterisk) is visualized at end-inspiration
Protocol
During PS weaning initiated by the primary ICU team, the US technician imaged the diaphragm. Diaphragms were visualized with ultrasound at PS settings of 15/5 cm of H2O, 10/5 cm of H2O, and 5/5 cm of H2O; the location of the probe on the patient was marked to minimize variability of the imaging window. Patients were maintained on the indicated PS for at least 5 min prior to US imaging. The primary ICU team extubated patients when able to tolerate PS of 10/5 cm of H2O or less for at least 2 h without exhibiting clinical signs of respiratory fatigue (increase in respiratory rate >30 breaths/min, heart rate >120 beats/min, SBP <90 mmHg or >180 mmHg, presence of confusion, agitation, diaphoresis, cyanosis, or evidence of increasing respiratory effort). All decisions to extubate were made without knowledge of US results. Patients were followed until discharge or death. Demographics, clinical information, and other ventilator metrics were obtained by chart review. Etiologies of respiratory failure with an incidence of one were combined into a miscellaneous group that consisted of such causes as diffuse alveolar hemorrhage, hepatic encephalopathy, toxic ingestion, non-ST segment elevation myocardial infarction, angioedema, delirium tremens, Lemeir’s Syndrome, acute respiratory distress syndrome, metabolic acidosis, and metabolic encephalopathy.
Analysis
Demographics, clinical information, and ventilator metrics were all summarized with descriptive statistics and compared using either Mann–Whitney test (two-group comparisons, continuous variables), or Kruskal–Wallis test with Dunn’s post hoc multiple comparison test (three-group comparison, continuous variables), or Z-test (two-group comparison, proportions), or Chi-squared test (three-group comparison, proportions). Percent change of diaphragmatic thickness (Δtdi%) stratified by PS level and time to extubation compared using a two-way analysis of variance (ANOVA) with a Bonferroni post hoc test. Receiver operating characteristic (ROC) curves were created to identify the optimal Δtdi% threshold for extubation within 48 h of ultrasound. For each PS level, sensitivity, specificity, positive predictive value, and negative predicative value were calculated. P < 0.05 was considered statistically significant. All statistical analyses were conducted on GraphPad Prism (GraphPad Software Inc., La Jola, CA, USA) and SPSS (IBM, Armonk, NY, USA).
Results
Fifty-six patients were enrolled in the study group, but four were censored prior to the final analysis due to self-extubation (Fig. 2). Of the remaining 52 patients, 26 were extubated within 48 h of US and 19 remained intubated for a longer period. Additionally, seven patients were extubated within 48 h of ultrasound but required emergent reintubation within the following two days. Patients extubated within 48 h and patients extubated after 48 h (columns 2 and 3 in Table 1) did not significantly differ in age, gender, BMI, severity of illness, reason for intubation, and etiology of respiratory failure. There was no difference in the time to first PS weaning trial. As expected, patients who were unable to be extubated within 48 h had significantly longer total weaning time (p < 0.001), total ventilation time (p < 0.001), longer ICU stays (p = 0.028) and higher rates of in hospital mortality (p < 0.001).
All 52 patients had ultrasound measurements of diaphragmatic thickness at 5/5 cm of H2O and 10/5 cm of H2O while 36 had imaging at 15/5 cm of H2O. At pressure support of 5/5 cm of H2O patients who were able to be successfully extubated within 48 h of ultrasound had a 2.12-fold larger percent change in diaphragmatic thickness during the respiratory cycle compared with those who were unable to be extubated within 2 days (Fig. 3a). These same patients had a significant 1.96-fold increase in ∆tdi% on pressure support settings of 10/5 cm H2O. However, at pressure support of 15/5 cm of H2O the average ∆tdi% for each group did not statistically differ.
Δtdi% is a robust predictor of extubation success within 48 h of ultrasound for pressure support levels up to 10/5. a Patients extubated within 48 h of ultrasound had significantly greater Δtdi% at 5/5 cm H2O and 10/5 cm H2O. At 15/5 cm H2O there was no significant difference between the two groups. b ROC curves of Δtdi% as a predictor of time to successful extubation showing 20 % as the optimal cut off at 5/5 cm H2O and 10/5 cm H2O. At 15/5 cm H2O, the optimal Δtdi% cutoff was 10 %
Receiver operating characteristic (ROC) curves were created at each level of pressure support to establish the optimal ∆tdi% threshold for predicting extubation within 48 h (Fig. 3b). As expected, ∆tdi% measured at 5/5 cm of H2O and 10/5 cm of H2O was equally robust in its ability to predict extubation (AUC 0.864 and 0.824, respectively). However, the area under the ROC curve for ∆tdi% at PS 15/5 cm of H2O was significantly less (AUC 0.667). Based on the ROC curve, ∆tdi% at PS 5/5 cm of H2O and 10/5 cm of H2O had an optimal cut off of 20 %, which corresponded with sensitivity of 84.6 and 88.9 % and specificity of 79.0 and 75.0 %, respectively. At PS 15/5 cm of H2O, the optimal cutoff was 10 % which had markedly reduced sensitivity and specificity (Table 2). Stratifying the cohort by reason for intubation demonstrated that ∆tdi% predicted extubation success more often in patients intubated for respiratory failure (hypoxic or hypercapnic) than those intubated for airway protection at all levels of pressure support (Table 2, sensitivity 94.4 % and specificity 82.5 % vs. sensitivity 75.0 % and specificity 33.3 %).
To better understand how diaphragmatic ultrasound compares to established extubation metrics, we compared ∆tdi% with RSBI. The ∆tdi% had a significant negative linear correlation with RSBI at a PS of 5/5 cm H2O (p = 0.025) but not at PS of 10/5 cm H2O or 15/5 cm H2O (Supplementary Fig. 1A–C). Using an RSBI cutoff of 105 breaths/min/L, RSBI had similar sensitivity but markedly lower specificity when compared with ∆tdi% at PS 5/5 cm H2O (Supplementary Table 1). At higher levels of PS, ∆tdi% was a far better predictor of extubation success within 48 h. The combination of RSBI and ∆tdi% was not significantly better than ∆tdi% alone.
Seven study patients extubated within 48 h required emergent reintubation within the following two days. These subjects did not differ from the prior two groups with regards to their demographics, severity of illness, reason for intubation, or etiology of respiratory failure (Table 1). However, they did have significantly longer ICU stay compared to those extubated within 48 h (p < 0.001). 85.7 % (six out of seven) of these patients had ∆tdi% less than the extubation threshold of 20 % (Fig. 4)
Discussion
Ultrasound is a readily available, easy to use, and non-invasive imaging modality that is commonly used for procedures and clinical evaluation in the modern ICU [18]. Recently, US measurements have been used to assess diaphragmatic function and predict extubation success [15, 18, 19]. We establish Δtdi% as a robust and specific predictor of successful extubation within 48 h of US measurement at PS levels as high as 10/5 cm of H2O in the general ICU population. At PS 15/5 cm of H2O there was no significant difference between the groups extubated within 48 h and those extubated after 48 h, and therefore, Δtdi% had no predictive capacity. This observation suggests that ∆tdi% could predict extubation outcomes at PS levels as great as 10/5 cm of H2O but not at 15/5 cm of H2O. Δtdi% better predicts extubation outcomes in patients who were intubated for respiratory failure as compared to those intubated for airway protection. Finally, we demonstrate that emergent reintubations occurred when the diaphragm failed to thicken sufficiently (i.e., Δtdi% was less than the proposed extubation threshold of 20 %).
Our study extends prior work that indicated that changes in diaphragm thickness measured at PS 5/5 cm of H2O or on T-piece can be used to predict extubation outcomes. DiNino et al. showed that Δtdi% of 30 % at initiation of weaning predicted extubation success of ICU patients [18]. A subsequent study by Ferrari et al. established that a Δtdi% of 36 % predicted successful weaning in patients requiring long-term ventilator support [19]. We show that a Δtdi% of 20 % or higher measured at PS up to 10/5 cm of H2O accurately predicted successful extubation within the next 48 h. Our Δtdi% threshold is less than those of the previous studies for several technical reasons. First, our study utilized an image processing program (ImageJ, NIH, Bethesda, MD, USA) for the analysis which achieved greater accuracy and precision than those achieved in previous studies. Furthermore, diaphragm thickness was measured at the inner most part of the pleura and peritoneum compared to the midline of these structures as in other studies. This variability reveals the need for a standard protocol before Δtdi% can be utilized globally to predict extubation success.
Interestingly, the predictive power of ∆tdi% drastically decreases above pressure supports of 10/5 cm of H2O. We hypothesize that at low levels of PS the ventilator supplies only enough support to overcome only the intrinsic resistance of the ventilatory circuit. To generate adequate spontaneous breathing, the diaphragm must maximally contract resulting in a large change in thickness [20, 21]. At PS greater than 10/5 cm of H2O, the ventilator offsets the work of breathing resulting in submaximal diaphragmatic contraction [16, 22]. This added support does not allow for true assessment of the diaphragms capabilities when mechanical support is removed, and hence cannot reliably predict extubation outcomes.
Δtdi% at a PS of 5/5 cm H2O correlates with RSBI but both metrics provide very different information about the respiratory system. RSBI represents the combined function of the diaphragm and accessory muscles while direct visualization of the diaphragm and calculation of Δtdi% evaluates diaphragm activation alone. By excluding the impact of the accessory muscles, which are unlikely to support unassisted respiration for a significant amount of time, Δtdi% has much higher specificity than RSBI (79.0 vs. 42.1 %) [20, 21]. This increased specificity results in fewer premature extubations and subsequent reintubations. Consistent with this, all but one patient reintubated within 48 h of extubation were deemed appropriate for extubation by the RSBI but six of the seven would not have been extubated using our proposed cutoff of Δtdi%.
A potential limitation of the study is the sample size. While adequate to detect changes in the main cohort, it did not have a sufficient size to allow for subgroup analysis. Specifically, with only seven patients requiring reintubation we did not have an adequate sample size to confirm the trend that reintubated patients had an average Δtdi% less than the proposed extubation threshold. Including patients with intracranial hemorrhage and stroke would have increased our sample size; however, successful extubation of such patients is more reliant on mental status than diaphragmatic strength and would have diluted the results of the study.
We conclude that ultrasound measurements of Δtdi% obtained at PS up to 10/5 cm of H2O can predict extubation success in the next 48 h. This is important since some patients are unable to wean on a PS of 5/5 cm H2O. This study bridges the predictors described in prior studies to today’s clinical practice. Furthermore, it establishes a 48-h window during which successful extubation can be expected. This result opens the possibility of a novel weaning protocol where patients undergo daily diaphragmatic ultrasound during PS support weans until Δtdi% is greater than 20 %. At that time successful extubation can be expected in the next 48 h. Incorporation of Δtdi% into established weaning protocols may improve a clinician’s predictive power and thereby reduce unnecessarily prolonged intubations and prevent emergent reintubations.
References
Hudson MB, Smuder AJ, Nelson WB, Bruells CS, Levine S, Powers SK (2012) Both high level pressure support ventilation and controlled mechanical ventilation induce diaphragm dysfunction and atrophy. Crit Care Med 40(4):1254–1260
Epstein SK, Ciubotaru RL (1998) Independent effects of etiology of failure and time to reintubation on outcome for patients failing extubation. Am J Respir Crit Care Med 158(2):489–493
MacIntyre NR, Cook DJ, Ely EW Jr et al (2001) Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; The American Association for Respiratory Care; and The American College of Critical Care Medicine. Chest 120(6 suppl):375S–395S
Esteban A, Anzueto A, Frutos F et al (2002) Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 287(3):345–355
Stroetz RW, Hubmayr RD (1995) Tidal volume maintenance during weaning with pressure support. Am J Resp Crit Care Med 152(3):1034–1040
Krieger BP, Ershowsky PF, Becker DA, Gazeroglu HB (1989) Evaluation of conventional criteria for predicting successful weaning from mechanical ventilatory support in elderly patients. Crit Care Med 17(9):858–861
Conti G, Montini L, Pennisi MA et al (2004) A prospective, blinded evaluation of indexes proposed to predict weaning from mechanical ventilation. Intensive Care Med 30(5):830–836
Yang KL, Tobin MJ (1991) A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med 324(21):1445–1450
Meade M, Guyatt G, Cook D et al (2001) Predicting success in weaning from mechanical ventilation. Chest 120:400S–424S
Lee KH, Hui KP, Chan TB, Tan WC, Lim TK (1994) Rapid shallow breathing (frequency-tidal volume ratio) did not predict extubation outcome. Chest 105(2):540–543
McCool FD, Conomos P, Benditt JO, Cohn D, Sherman CB, Hoppin FG Jr (1997) Maximal inspiratory pressures and dimensions of the diaphragm. Am J Respir Crit Care Med 155(4):1329–1334
Cohn DB, Benditt JO, Eveloff SE, McCool FD (1997) Diaphragm thickening during inspiration. J Appl Physiol 83(1):291–296
Lerolle N, Guerot E, Dimassi S et al (2009) Ultrasonographic diagnostic criterion for severe diaphragmatic dysfunction after cardiac surgery. Chest 135(2):401–407
Gottesman E, McCool FD (1997) Ultrasound evaluation of the paralyzed diaphragm. Am J Respir Crit Care Med 155(5):1570–1574
Kim WY, Suh HJ, Hong SB, Koh Y, Lim CM (2011) Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med 39(12):2627–2630
Umbrello M, Formenti P, Longhi D et al (2015) Diaphragm ultrasound as indicator of respiratory effort in critically ill patients undergoing assisted mechanical ventilation: a pilot clinical study. Crit Care 19:161
Miller WT, Talman EA (1967) Subphrenic abscess. Am J Roentgenol Radium Ther Nucl Med 101(4):961–969
DiNino E, Gartman EJ, Sethi JM, McCool FD (2014) Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 69(5):423–442
Ferrari G, De Filippi G, Elia F, Panero F, Volpicelli G, Apra F (2014) Diaphragm ultrasound as a new index of discontinuation from mechanical ventilation. Crit Ultrasound J. 6(1):8
Hershenson MB, Kikuchi Y, Tzelepis GE, McCool FD (1989) Preferential fatigue of the rib cage muscles during inspiratory resistive loaded ventilation. J Appl Physiol 66(2):750–754
Hershenson MB, Kikuchi Y, Loring SH (1988) Relative strengths of the chest wall muscles. J Appl Physiol 65(2):852–862
Vivier E, Mekontso Dessap A, Dimassi S et al (2012) Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med 38(5):796–803
Acknowledgments
We would like to acknowledge Dr. Glenn Eiger for his insightful comments during the planning stages of this study.
Authors' Contribution
JM is the guarantor of the content of this manuscript and contributed to study design, data analysis, and revisions to the manuscript. SB contributed to study design, data collection, data analysis, and drafted the manuscript. DW contributed to study design, data collection, data analysis, and revisions to the manuscript. KT contributed to data collection, and revisions to the manuscript. FDM contributed to study design and revisions to the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Ethical Approval
Einstein Medical Center Philadelphia IRB #4541.
Electronic supplementary material
Below is the link to the electronic supplementary material.
408_2016_9911_MOESM2_ESM.tiff
Supplementary material 2. Δtdi% Correlated with RSBI at PS 5/5 cm H2O (A) but not at PS 10/5 cm H2O (B) or 15/5 cm H2O (TIFF 16348 kb)
Rights and permissions
About this article
Cite this article
Blumhof, S., Wheeler, D., Thomas, K. et al. Change in Diaphragmatic Thickness During the Respiratory Cycle Predicts Extubation Success at Various Levels of Pressure Support Ventilation. Lung 194, 519–525 (2016). https://doi.org/10.1007/s00408-016-9911-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-016-9911-2