Abstract
Purpose
We hypothesized that: (1) intraabdominal hypertension increases pulmonary inflammatory and fibrogenic responses in acute lung injury (ALI); (2) in the presence of intraabdominal hypertension, higher tidal volume reduces lung damage in extrapulmonary ALI, but not in pulmonary ALI.
Methods
Wistar rats were randomly allocated to receive Escherichia coli lipopolysaccharide intratracheally (pulmonary ALI) or intraperitoneally (extrapulmonary ALI). After 24 h, animals were randomized into subgroups without or with intraabdominal hypertension (15 mmHg) and ventilated with positive end expiratory pressure = 5 cmH2O and tidal volume of 6 or 10 ml/kg during 1 h. Lung and chest wall mechanics, arterial blood gases, lung and distal organ histology, and interleukin (IL)-1β, IL-6, caspase-3 and type III procollagen (PCIII) mRNA expressions in lung tissue were analyzed.
Results
With intraabdominal hypertension, (1) chest-wall static elastance increased, and PCIII, IL-1β, IL-6, and caspase-3 expressions were more pronounced than in animals with normal intraabdominal pressure in both ALI groups; (2) in extrapulmonary ALI, higher tidal volume was associated with decreased atelectasis, and lower IL-6 and caspase-3 expressions; (3) in pulmonary ALI, higher tidal volume led to higher IL-6 expression; and (4) in pulmonary ALI, liver, kidney, and villi cell apoptosis was increased, but not affected by tidal volume.
Conclusions
Intraabdominal hypertension increased inflammation and fibrogenesis in the lung independent of ALI etiology. In extrapulmonary ALI associated with intraabdominal hypertension, higher tidal volume improved lung morphometry with lower inflammation in lung tissue. Conversely, in pulmonary ALI associated with intraabdominal hypertension, higher tidal volume increased IL-6 expression.
Similar content being viewed by others
Introduction
Intraabdominal hypertension may be associated with pulmonary and extrapulmonary acute lung injury and acute respiratory distress syndrome (ALI/ARDS) [1], compressing the thoracic cavity, increasing pleural pressure [2, 3], and causing alveolar edema [2] and atelectasis [3]. Low tidal volume (V T) ventilation and airway plateau pressure below 30 cmH2O have been shown to reduce ventilator-associated lung injury (VALI) and mortality [4] in a mixed population of ALI/ARDS patients. However, in the presence of intraabdominal hypertension, low VT may promote alveolar derecruitment, depending on the degree of inspiratory transpulmonary pressure [5].
Such effects could be more pronounced in extrapulmonary ALI, which is characterized by less consolidation and higher potential of recruitment [1, 6–8]. Therefore, in extrapulmonary ALI associated with intraabdominal hypertension, high V T may lead to increased inspiratory transpulmonary pressure and better recruitment, reducing the risk of VALI, while protective ventilation may further increase atelectasis and worsen lung damage. Furthermore, VALI may lead to activation of inflammatory mediators and increased damage to peripheral organs [9].
In the present study, we hypothesized that (1) intraabdominal hypertension increases pulmonary inflammatory and fibrogenic responses in ALI; and (2) in the presence of intraabdominal hypertension, higher V T may reduce VALI in extrapulmonary, but not in pulmonary ALI. We compared the effects of protective (6 ml/kg) and higher V T (10 ml/kg) on arterial blood gases, respiratory system, lung and chest wall mechanics, lung histology, inflammatory and fibrogenic mediator mRNA expression, and apoptosis in lung and distal organs in experimental pulmonary and extrapulmonary ALI with or without intraabdominal hypertension.
Materials and methods
Detailed methods are described in the electronic supplementary material (ESM) accompanying this article and briefly summarized here.
Animal preparation and experimental protocol
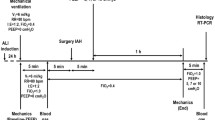
This study was approved by the Health Sciences Center Ethics Committee at the Federal University of Rio de Janeiro, Brazil. Forty-eight Wistar rats (300–350 g) were randomly assigned to two groups. Animals received Escherichia coli lipopolysaccharide [O55:B5] either intratracheally (200 μg) (pulmonary ALI, ALIp; n = 24) or intraperitoneally (1,000 μg) (extrapulmonary ALI, ALIexp; n = 24), suspended in saline solution with total volumes equal to 20 and 1,000 μl, respectively. These doses of E. coli lipopolysaccharide yielded a similar 1.5-fold increase in lung static elastance in ALIp and ALIexp groups. Twenty-four hours after ALI induction, rats were sedated (diazepam 5 mg intraperitoneally), anesthetized (thiopental sodium 20 mg/kg intraperitoneally), and tracheotomized. A polyethylene catheter (PE-50) was introduced into the carotid artery for blood sampling and monitoring of mean arterial pressure. Animals were paralyzed (vecuronium bromide 2 mg/kg, intravenously) and mechanically ventilated (Servo-i, MAQUET, Sweden) in volume-controlled ventilation with: V T = 6 ml/kg, respiratory rate (RR) = 80 breaths/min, inspiratory-to-expiratory ratio = 1:2, fraction of inspired oxygen (FiO2) = 1.0, and positive end-expiratory pressure (PEEP) = 0 cmH2O (ZEEP) during 5 min. Blood (300 μl) was drawn into a heparinized syringe to determine arterial oxygen partial pressure (PaO2), arterial carbon dioxide partial pressure (PaCO2), and arterial pH (i-STAT, Abbott Laboratories, IL, USA) (Baseline-ZEEP). Mechanical ventilation was set as follows: RR = 80 breaths/min, PEEP = 5 cmH2O, and FiO2 = 0.4. Each subgroup was randomized to ventilation with V T of 6 or 10 ml/kg (n = 12/group).
Respiratory system, lung, and chest wall mechanics were measured (Baseline). In each group, the animals were further randomized, and intraabdominal hypertension (15 mmHg) was induced by laparotomy and placement of cotton dressings or not (normal intraabdominal pressure) (n = 6/group). Intraabdominal hypertension was maintained at this level throughout the experiment. Manipulation of the normal intraabdominal pressure group was identical, except for the use of dressings. Mechanical properties of the respiratory system, lung, and chest wall were measured again immediately after intraabdominal hypertension induction or sham surgery (After-surgery), and after 1 h ventilation (End). Following this step, FiO2 was set at 1.0. After 5 min, arterial blood gases were analyzed at PEEP = 5 cmH2O. Animals were then euthanized. IL-6, IL-1β, caspase-3, and PCIII mRNA expressions were measured in lung tissue. A schematic flow chart of study design and the timeline representation of the procedure are shown in the electronic supplementary material (Figs. ESM1, ESM2).
Data acquisition and processing
Airflow and tracheal pressure were continuously recorded using LabVIEW® software (National Instruments, Austin, TX, USA). Tidal volume was obtained by integration of airflow. Changes in esophageal pressure, which reflect chest wall pressure, were measured. Transpulmonary pressures were calculated during inspiration and expiration as the difference between airway and esophageal pressures. Respiratory system, lung, and chest wall static elastance were measured by the end-inflation occlusion technique [8, 10]. Furthermore, respiratory system plateau pressure, delta transpulmonary pressure (difference between end-inspiratory and end-expiratory transpulmonary pressure), and delta chest wall pressure were computed.
Lung and distal organ histology
Light microscopy
At the end of the experiments (End-PEEP), lungs, liver, kidneys, and small intestine were removed, fixed (3% buffered formaldehyde), paraffin embedded, and stained with hematoxylin-eosin. Fraction areas of the lung occupied by hyperinflated structures, and collapsed and normal lung areas were determined by the point-counting technique [11] across ten random, non-coincident microscopic fields [8, 12, 13].
Transmission electron microscopy
Three slices measuring 2 × 2 × 2 mm were cut from three different segments of the left lung and diaphragm, and fixed for electron microscopy (JEOL 1010 Transmission Electron Microscope, Tokyo, Japan). Each electron microscopy image (15/animal) was analyzed for damage to the alveolar capillary membrane, type II pneumocyte, and endothelial cells. Pathological findings were graded according to a 5-point semi-quantitative severity-based scoring system as: 0 = normal lung parenchyma, 1 = 1–25%, 2 = 26–50%, 3 = 51–75%, and 4 = 76–100% of examined tissue [8, 10, 14].
Apoptosis assays
Apoptotic cells of lung, kidney, liver, and small intestine villi were quantified using terminal deoxynucleotidyl transferase biotin-dUTP nick end Labeling (TUNEL) assay in a blinded fashion by two pathologists. A 5-point semi-quantitative severity-based scoring system was used to assess apoptosis: 0 = normal lung parenchyma; 1 = 1–25%; 2 = 26–50%; 3 = 51–75%; 4 = 76–100% of examined tissue [13, 15].
IL-6, IL-1β, caspase-3, and PCIII mRNA expressions
Quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) was performed to measure the relative levels of expression of interleukin (IL)-6, IL-1β, caspase-3, and type III procollagen genes in ALIp and ALIexp [10]. For ALIp, each set of experiments contained: ALIp-nIAP-V T6, ALIp-nIAP-V T10, ALIp-IAH-V T6, and ALIp-IAH-V T10. Each gene was studied in triplicate for each animal. For the set of ALIp, ALIp-nIAP-V T6 was taken as 1. The entire set of experiments was performed four times, one for each of four animals in each group (n = 4). The same was performed for ALIexp (ALIexp-nIAP-V T6, ALIexp-nIAP-V T10, ALIexp-IAH-V T6, and ALIexp-IAH-V T10), and again ALIexp-nIAP-V T6 was taken as 1. For each sample, the expression of each gene was normalized to housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression using the 2−ΔΔCt method, where ΔCt = Ct, reference gene-Ct, target gene.
Statistical analysis
In each ALI group, the effects of different V T with or without intraabdominal hypertension were assessed with two-way ANOVA or two-way ANOVA on ranks followed by Tukey’s test. Parametric data are expressed as mean ± SD, while non-parametric data are expressed as median (interquartile range). All tests were performed using the Sigma Stat 3.1 statistical software package (Jandel Corporation, San Rafael, CA, USA). Significance was established at p < 0.05.
Results
Intraabdominal hypertension yielded greater chest wall static elastance compared to normal intraabdominal pressure, with no significant changes in lung and respiratory system static elastance (Table ESM1; Fig. ESM5). All ALI animals showed alveolar collapse (Figs. 1, 2), cytoplasmatic degeneration of type II pneumocytes (PII), endothelial injury, and alveolar capillary damage (Table 1; Fig. ESM6). In ALI groups, intraabdominal hypertension and V T did not affect lung cell apoptosis (Table 2). IL-6, IL-1β, PCIII, and caspase-3 mRNA expressions were more elevated in intraabdominal hypertension than in normal intraabdominal pressure, independent of ALI etiology (Fig. 3).
Photomicrographs of lung parenchyma stained with hematoxylin-eosin in pulmonary (p) and extrapulmonary (exp) acute lung injury (ALI) associated with intraabdominal hypertension (IAH) or not (nIAP), ventilated with a tidal volume of 6 ml/kg (V T6) or 10 ml/kg (V T10). Photomicrographs are representative of data obtained from lung sections derived from six animals and were taken at an original magnification of ×200. Note the presence of alveolar collapse (arrows) and interstitial edema (asterisk)
The volume fraction of the lung occupied by normal pulmonary areas, collapsed alveoli, and hyperinflated structures in pulmonary (p) and extrapulmonary (exp) acute lung injury (ALI), associated with intraabdominal hypertension (IAH) or not (nIAP), ventilated with tidal volume of 6 ml/kg (V T6) or 10 ml/kg (V T10). Each bar represents the mean ± SD of six rats in each group. †Significantly different from nIAP (p < 0.05). ‡Significantly different from V T6 (p < 0.05)
Real-time polymerase chain reaction analysis of interleukin (IL)-6, IL1-β, caspase-3, and type III procollagen (PCIII) mRNA expressions in lung tissue of animals with pulmonary (p) and extrapulmonary (exp) acute lung injury (ALI) associated with intraabdominal hypertension (IAH) or not (nIAP), ventilated with a tidal volume of 6 ml/kg (V T6) or 10 ml/kg (V T10). Each bar represents the mean ± SD of four rats in each group. Data are normalized to GAPDH expression. The y axis represents fold changes in mRNA expression compared with the level detected in ALIp-nIAP-V T6 (for ALIp) and ALIexp-nIAP-V T6 (for ALIexp), which were considered as 1. †Significantly different from nIAP (p < 0.05). ‡Significantly different from V T6 (p < 0.05)
Extrapulmonary ALI
In ALIexp, intraabdominal hypertension resulted in higher alveolar collapse at V T6 (Figs. 1, 2). The volume fraction of alveolar collapse was lower with V T10 compared to V T6 (Fig. 2). Endothelial cell injury was more prevalent in ALIexp than ALIp (Table 1). Alveolar capillary membrane and endothelial cell damages were not affected by either intraabdominal hypertension or V T (Table 1, Fig. ESM6). V T10 reduced IL-6 and caspase-3, but not IL-1β and PCIII mRNA expressions (Fig. 3) in the presence of intraabdominal hypertension. Conversely, with normal intraabdominal pressure, V T did not alter IL-6, IL-1β, PCIII, and caspase-3 mRNA expressions.
Pulmonary ALI
In ALIp, neither intraabdominal hypertension nor V T affected the volume fraction of collapsed alveoli (Figs. 1, 2). Epithelial cell damage was more pronounced in ALIp than ALIexp. Alveolar capillary membrane injury was more intense at V T6, and the degree of endothelial cell damage was greater at V T10. The degree of cell apoptosis in liver, kidney, and villi was higher with intraabdominal hypertension compared to normal intraabdominal pressure, independent of V T (Table 2). In the presence of intraabdominal hypertension, IL-6 mRNA expression was higher in V T10 compared to V T6, but no significant differences were observed in IL-1β, PCIII, and caspase-3 mRNA expressions (Fig. 3). In the presence of normal intraabdominal pressure, V T10 led to higher IL-6 and caspase-3 mRNA expressions compared to V T6, while IL-1β and PCIII mRNA expressions were not affected by V T (Fig. 3).
Discussion
In the present study, we investigated the effects of intraabdominal hypertension as well as the impact of protective (6 ml/kg) and higher (10 ml/kg) V T in experimental pulmonary and extrapulmonary ALI with or without intraabdominal hypertension. In the presence of intraabdominal hypertension, we found that: (1) chest wall static elastance, IL-6, IL-1β, PCIII, and caspase-3 mRNA expressions in lung tissue were more pronounced as compared to normal intraabdominal pressure in both ALI groups; (2) in ALIexp, higher V T (10 ml/kg) was associated with decreased atelectasis and lower IL-6 and caspase-3 mRNA expressions in lung tissue; (3) in ALIp, higher V T led to higher IL-6 mRNA expression in lung tissue; (4) the degree of liver, kidney, and villi cell apoptosis was increased in ALIp, but not affected by V T.
To assess the effects of intraabdominal hypertension on ALIp and ALIexp, we chose models with similar deterioration in lung mechanics (Table ESM1) and oxygenation (Table ESM2). Intraabdominal hypertension was induced by inserting cotton dressings into the abdominal cavity until intraabdominal pressure of 15 mmHg was achieved, because, as previously described, intraperitoneal fluid infusion has the potential to be absorbed and therefore interfere with the pathophysiological response to intraabdominal hypertension [16], while the use of air inflation with CO2 may represent an additional physiologic variable related to the body’s response to intraabdominal hypertension [17]. The advantage of our intraabdominal hypertension model was the maintenance of high intraabdominal pressure and the absence of any side effects related to the gas inflated into the peritoneum. Although these animals may be ventilated with different combinations of V T and PEEP, we used V T = 6 ml/kg and 10 ml/kg, which have been considered to be in a safe range [18, 19], associated with PEEP = 5 cmH2O. Additionally, in the presence of intraabdominal hypertension, the increase in PEEP may lead to negative hemodynamic effects, as well as fluid and/or vasoactive drug requirements to stabilize hemodynamics. IL-6, IL-1β, and caspase-3 mRNA expressions in lung were analyzed because of their role as mediators in the pathogenesis of ventilator induced lung injury [20]. The expression of PCIII mRNA was evaluated in lung tissue because it is the first collagen to be remodeled in the development/course of lung fibrogenesis [21], and also an early marker of lung parenchyma remodeling [13, 14, 22].
The impact of intraabdominal hypertension on acute lung injury
As previously reported, intraabdominal hypertension causes an increase in chest wall static elastance [2, 23–25]. In our study, during ventilation with V T6, intraabdominal hypertension did not affect lung static elastance in either ALI group. Lung static elastance was not correlated to lung morphology. This may be explained by a greater alveolar collapse and the presence of adhesive atelectasis and consolidation in ALIp [8]. In short, the extent and characteristics of atelectasis at baseline in ALIp may have limited the effects of intraabdominal hypertension, preventing further alveolar collapse. Conversely, intraabdominal hypertension resulted in increased alveolar collapse mainly in ALIexp ventilated with V T6.
Intraabdominal hypertension increased mRNA expression of IL-6, IL-1β, PCIII, and caspase-3 independent of ALI etiology. Intraabdominal hypertension is known to release pro-inflammatory cytokines, leading to neutrophil infiltration [26, 27]. However, the effects of intraabdominal hypertension on the fibrogenic process as well as caspase-3 have not been investigated. The increase in PCIII may be related to the increase in inflammatory mediators. Although caspase-3 expression was more elevated in intraabdominal hypertension, no significant changes were observed in the degree of apoptosis measured by TUNEL or electron microscopy. This could be explained by the fact that caspase-3 expression was evaluated at RNA instead of the protein level.
Our data suggest that the inflammatory response might not be related to the presence of alveolar collapse, but rather associated with alveolar-capillary barrier damage in ALIp with intraabdominal hypertension (Table 1). Therefore, the type of ALI seems to be deeply related to the type of effect exerted by intraabdominal hypertension on lung morphology and biochemical response.
The effects of ALI associated with intraabdominal hypertension on inflammatory response might be ascribed to changes in regional chest wall mechanics, which may yield different local transpulmonary pressures [1] resulting in inhomogeneous distribution of ventilation and/or re-opening and closing of atelectatic lung regions, with different stress and strain; increase in thoracic blood volume [28]; reduction in lymphatic drainage [29]; and development of systemic inflammation from the abdomen, affecting the lung [27]. Furthermore, in ALIexp, a greater inflammatory response from the abdomen could be expected in the presence of intraabdominal hypertension.
V T6 versus V T10 in pulmonary and extrapulmonary ALI with intraabdominal hypertension
A VT of 6 ml/kg has been recommended during protective lung ventilation to improve survival in patients with different clinical risk factors for ALI/ARDS [18, 30]. A V T of 10 ml/kg has been found not to significantly affect outcome as compared to 6 ml/kg [19]. However, no study so far has evaluated the response to different V T in ALIp and ALIexp associated with intraabdominal hypertension.
In the present study, V T setting influenced respiratory function and lung injury depending on ALI etiology. In ALIp with or without intraabdominal hypertension, V T did not significantly affect the amount of alveolar collapse. However, the use of V T10 increased lung injury, which may be related to the presence of alveolar consolidation and/or sticky alveolar collapse, likely requiring higher inspiratory transpulmonary pressures to reopen. These data are in line with previous works showing lung injury at higher V T [31–33] and peak inspiratory pressure [34, 35].
In ALIexp associated with intraabdominal hypertension, V T6 increased while V T10 decreased atelectasis. The reduction in atelectasis led to oxygenation improvement and may be related to higher end-inspiratory transpulmonary pressure at V T10 and different characteristics of alveolar collapse. Higher V T led to a reduction in inflammatory mediators in the lung tissue.
Our data suggest that even if higher end-inspiratory transpulmonary pressure were reached with V T10, the reduction in alveolar collapse would play a major role in minimizing lung injury in the presence of intraabdominal hypertension. Similar effects have been reported with other ALI models [31, 36]. Although it has been accepted that lung injury correlates with variation in transpulmonary pressure [37], under certain conditions this may not be valid. In this context, in the presence of intraabdominal hypertension, the higher transpulmonary pressure between end-inspiration and end-expiration at V T10 compared to V T6 was not associated with increased lung injury.
In the present study, we were not able to measure strain, i.e., the ratio between tidal volume and aerated lung volume at end-expiration. However, the percentage of aerated areas may be regarded as a surrogate of aerated lung volume. Since V T did not change aeration in ALIp associated with intraabdominal hypertension, we may hypothesize that V T10 was associated with increased strain of lung parenchyma. Conversely, in ALIexp associated with intraabdominal hypertension, V T10 promoted an increase in aerated lung volume, suggesting that strain decreased or remained unaltered.
At VT6, intraabdominal hypertension increased peripheral organ damage mainly in ALIp, perhaps because of the greater initial peripheral organ damage in ALIexp. This is expected as a result of endotoxin release in the blood. Moreover, intraabdominal hypertension may evolve to abdominal compartment syndrome. Increased intraabdominal pressure reduces blood flow to intra-abdominal organs [38], with consequent apoptosis of enterocytes [39] and hepatocytes [40]. However, independent of VT, liver, kidney, and villi cell apoptosis were increased with intraabdominal hypertension.
Limitations
This study has several limitations: (1) we used specific models of ALIp and ALIexp induced by intratracheal and intraperitoneal endotoxin injection. Our results may not be extrapolated to other ALI models in small or large animals; (2) we did not assess possible long-term effects of V T and intraabdominal hypertension; (3) a specific level of intraabdominal hypertension (15 mmHg) and V T (6 vs. 10 ml/kg) were used; (4) PEEP was not individually titrated [2, 13]; rather, a fixed PEEP level (5 cmH2O) was applied. It is therefore possible that the observed increase in atelectasis and changes in biochemical parameters might reflect the inability of the combination between PEEP and VTs to open the lung or the failure of PEEP to keep the alveoli open at the present levels of intraabdominal hypertension. We cannot rule out that different results could have been obtained at higher PEEP levels and/or if recruitment maneuvers were applied; (5) respiratory rate was kept constant, since its increase has been reported to be associated with the release of inflammatory mediators [12]; (6) inflammatory mediators, apoptosis, and fibrogenetic response were measured in lung tissue but not in the blood; (7) anti-inflammatory responses were not evaluated; and (8) since the observation time was relatively short (1 h mechanical ventilation), the expression of mediators was quantified using RT-PCR instead of ELISA. In this line, it is well known that 1 h is sufficient time to produce changes in mRNA expression, but not to significantly change the level of protein [8, 10, 12–14, 22].
Conclusions
Intraabdominal hypertension increased inflammation and fibrogenesis in the lung independent of ALI etiology. With intraabdominal hypertension, V T10 improved lung morphometry with lower inflammation in lung tissue compared to V T6 in ALIexp; conversely, it increased IL-6 expression in ALIp. Thus, V T10 is one possible strategy to be proposed in the presence of intraabdominal hypertension, but other strategies deserve further investigation.
References
Rocco PR, Pelosi P (2008) Pulmonary and extrapulmonary acute respiratory distress syndrome: myth or reality? Curr Opin Crit Care 14:50–55
Quintel M, Pelosi P, Caironi P, Meinhardt JP, Luecke T, Herrmann P, Taccone P, Rylander C, Valenza F, Carlesso E, Gattinoni L (2004) An increase of abdominal pressure increases pulmonary edema in oleic acid-induced lung injury. Am J Respir Crit Care Med 169:534–541
Pelosi P, Luecke T, Rocco PR (2011) Chest wall mechanics and abdominal pressure during general anaesthesia in normal and obese individuals and in acute lung injury. Curr Opin Crit Care 17:72–79
Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P (2009) Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med 151:566–576
Sarge T, Talmor D (2008) Transpulmonary pressure: its role in preventing ventilator-induced lung injury. Minerva Anestesiol 74:335–339
Menezes SL, Bozza PT, Neto HC, Laranjeira AP, Negri EM, Capelozzi VL, Zin WA, Rocco PR (2005) Pulmonary and extrapulmonary acute lung injury: inflammatory and ultrastructural analyses. J Appl Physiol 98:1777–1783
Santos FB, Nagato LK, Boechem NM, Negri EM, Guimarães A, Capelozzi VL, Faffe DS, Zin WA, Rocco PR (2006) Time course of lung parenchyma remodeling in pulmonary and extrapulmonary acute lung injury. J Appl Physiol 100:98–106
Riva DR, Oliveira MB, Rzezinski AF, Rangel G, Capelozzi VL, Zin WA, Morales MM, Pelosi P, Rocco PR (2008) Recruitment maneuver in pulmonary and extrapulmonary experimental acute lung injury. Crit Care Med 36:1900–1908
Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V, Cutz E, Liu M, Keshavjee S, Martin TR, Marshall JC, Ranieri VM, Slutsky AS (2003) Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 289:2104–2112
Silva PL, Cruz FF, Fujisaki LC, Oliveira GP, Samary CS, Ornellas DS, Maron-Gutierrez T, Rocha NN, Goldenberg R, Garcia CS, Morales MM, Capelozzi VL, Gama de Abreu M, Pelosi P, Rocco PR (2010) Hypervolemia induces and potentiates lung damage after recruitment maneuver in a model of sepsis-induced acute lung injury. Crit Care 14:R114
Weibel ER (1990) Morphometry: stereological theory and practical methods. In: Dekker GJNY (ed) Models of lung disease––microscopy and structural methods. Marcel Dekker, New York, pp 199–247
Garcia CS, Abreu SC, Soares RM, Prota LF, Figueira RC, Morales MM, Capelozzi VL, Zin WA, Rocco PR (2008) Pulmonary morphofunctional effects of mechanical ventilation with high inspiratory air flow. Crit Care Med 36:232–239
Steimback PW, Oliveira GP, Rzezinski AF, Silva PL, Garcia CS, Rangel G, Morales MM, Silva Lapa E, JR Capelozzi VL, Pelosi P, Rocco PR (2009) Effects of frequency and inspiratory plateau pressure during recruitment manoeuvres on lung and distal organs in acute lung injury. Intensive Care Med 35:1120–1128
Pássaro CP, Silva PL, Rzezinski AF, Abrantes S, Santiago VR, Nardelli L, Santos RS, Barbosa CM, Morales MM, Zin WA, Amato MB, Capelozzi VL, Pelosi P, Rocco PR (2009) Pulmonary lesion induced by low and high positive end-expiratory pressure levels during protective ventilation in experimental acute lung injury. Crit Care Med 37:1011–1017
Oliveira GP, Oliveira MB, Santos RS, Lima LD, Dias CM, Ab’ Saber AM, Teodoro WR, Capelozzi VL, Gomes RN, Bozza PT, Pelosi P, Rocco PR (2009) Intravenous glutamine decreases lung and distal organ injury in an experimental model of abdominal sepsis. Crit Care 13:R74
Mutoh T, Lamm WJ, Embree LJ, Hildebrandt J, Albert RK (1992) Volume infusion produces abdominal distension, lung compression, and chest wall stiffening in pigs. J Appl Physiol 72:575–582
Kopernik G, Avinoach E, Grossman Y, Levy R, Yulzari R, Rogachev B, Douvdevani A (1998) The effect of a high partial pressure of carbon dioxide environment on metabolism and immune function of human peritoneal cells––Relevance to carbon dioxide pneumoperitoneum. Am J Obstet Gynecol 179:1503–1510
Eisner MD, Thompson T, Hudson LD, Luce JM, Hayden D, Schoenfeld D, Matthay MA (2001) Acute respiratory distress syndrome network efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 164:231–236
Stewart TE, Meade MO, Cook DJ, Granton JT, Hodder RV, Lapinsky SE, Mazer CD, McLean RF, Rogovein TS, Schouten BD, Todd TR, Slutsky AS (1998) Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure- and Volume-Limited Ventilation Strategy Group. N Engl J Med 338:355–361
Fanelli V, Mascia L, Puntorieri V, Assenzio B, Elia V, Fornaro G, Martin EL, Bosco M, Delsedime L, Fiore T, Grasso S, Ranieri VM (2009) Pulmonary atelectasis during low stretch ventilation: “open lung” versus “lung rest” strategy. Crit Care Med 37:1046–1053
Garcia CS, Rocco PR, Facchinetti LD, Lassance RM, Caruso P, Deheinzelin D, Morales MM, Romero PV, Faffe DS, Zin WA (2004) What increases type III procollagen mRNA levels in lung tissue: stress induced by changes in force or amplitude? Respir Physiol Neurobiol 144:59–70
Santiago VR, Rzezinski AF, Nardelli LM, Silva JD, Garcia CS, Maron-Gutierrez T, Ornellas DS, Morales MM, Capelozzi VL, Marini J, Pelosi P, Rocco PR (2010) Recruitment maneuver in experimental acute lung injury: the role of alveolar collapse and edema. Crit Care Med 38:2207–2214
Pelosi P, Croci M, Calappi E, Mulazzi D, Cerisara M, Vercesi P, Vicardi P, Gattinoni L (1996) Prone positioning improves pulmonary function in obese patients during general anesthesia. Anesth Analg 83:578–583
Pelosi P, Ravagnan I, Giurati G, Panigada M, Bottino N, Tredici S, Eccher G, Gattinoni L (1999) Positive end-expiratory pressure improves respiratory function in obese but not in normal subjects during anesthesia and paralysis. Anesthesiology 91:1221–1231
Henzler D, Hochhausen N, Bensberg R, Schachtrupp A, Biechele S, Rossaint R, Kuhlen R (2010) Effects of preserved spontaneous breathing activity during mechanical ventilation in experimental intra-abdominal hypertension. Intensive Care Med 36:1427–1435
Rezende-Neto JB, Moore EE, Melo de Andrade MV, Teixeira MM, Lisboa FA, Arantes RM, de Souza DG, da Cunha-Melo JR (2002) Systemic inflammatory response secondary to abdominal compartment syndrome: stage for multiple organ failure. J Trauma 53:1121–1128
Rezende-Neto JB, Moore EE, Masuno T, Moore PK, Johnson JL, Sheppard FR, Cunha-Melo JR, Silliman CC (2003) The abdominal compartment syndrome as a second insult during systemic neutrophil priming provokes multiple organ injury. Shock 20:303–308
Hofer CK, Zalunardo MP, Klaghofer R, Spahr T, Pasch T, Zollinger A (2002) Changes in intrathoracic blood volume associated with pneumoperitoneum and positioning. Acta Anaesthesiol Scand 46:303–308
Hedenstierna G, Lattuada M (2008) Lymphatics and lymph in acute lung injury. Curr Opin Crit Care 14:31–36
Rocco PR, Pelosi P, de Abreu MG (2010) Pros and cons of recruitment maneuvers in acute lung injury and acute respiratory distress syndrome. Expert Rev Respir Med 4:479–489
Saddy F, Oliveira GP, Garcia CS, Nardelli LM, Rzezinski AF, Ornellas DS, Morales MM, Capelozzi VL, Pelosi P, Rocco PR (2010) Assisted ventilation modes reduce the expression of lung inflammatory and fibrogenic mediators in a model of mild acute lung injury. Intensive Care Med 36:1417–1426
Carlton DP, Cummings JJ, Scheerer RG, Poulain FR, Bland RD (1990) Lung overexpansion increases pulmonary microvascular protein permeability in young lambs. J Appl Physiol 69:577–583
Bruhn A, Bugedo D, Riquelme F, Varas J, Retamal J, Besa C, Cabrera C, Bugedo G (2011) Tidal volume is a major determinant of cyclic recruitment-derecruitment in acute respiratory distress syndrome. Minerva Anestesiol 77:418–426
Dreyfuss D, Soler P, Basset G, Saumon G (1988) High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis 137:1159–1164
Hernandez LA, Peevy KJ, Moise AA, Parker JC (1989) Chest wall restriction limits high airway pressure-induced lung injury in young rabbits. J Appl Physiol 66:2364–2368
Spieth PM, Carvalho AR, Pelosi P, Hoehn C, Meissner C, Kasper M, Hübler M, von Neindorff M, Dassow C, Barrenschee M, Uhlig S, Koch T, de Abreu MG (2009) Variable tidal volumes improve lung protective ventilation strategies in experimental lung injury. Am J Respir Crit Care Med 179:684–693
Gattinoni L, Protti A, Caironi P, Carlesso E (2010) Ventilator-induced lung injury: the anatomical and physiological framework. Crit Care Med 38:S539–S548
Kotzampassi K, Paramythiotis D, Eleftheriadis E (2000) Deterioration of visceral perfusion caused by intra-abdominal hypertension in pigs ventilated with positive end-expiratory pressure. Surg Today 30:987–992
Gong G, Wang P, Ding W, Zhao Y, Li J (2009) The role of oxygen-free radical in the apoptosis of enterocytes and bacterial translocation in abdominal compartment syndrome. Free Radic Res 43:470–477
Mogilner JG, Bitterman H, Hayari L, Brod V, Coran AG, Shaoul R, Lurie M, Eldar S, Sukhotnik I (2008) Effect of elevated intra-abdominal pressure and hyperoxia on portal vein blood flow, hepatocyte proliferation and apoptosis in a rat model. Eur J Pediatr Surg 18:380–386
Acknowledgments
The authors would like to express their gratitude to Mr. Andre Benedito da Silva for animal care, Mrs. Ana Lucia Neves da Silva for her help with microscopy, Mrs. Moira Elizabeth Schottler and Ms. Claudia Buchweitz for their assistance in editing the manuscript, Prof. Ronir Raggio Luiz for revising the statistics, and MAQUET for supporting us technically.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Santos, C.L., Moraes, L., Santos, R.S. et al. Effects of different tidal volumes in pulmonary and extrapulmonary lung injury with or without intraabdominal hypertension. Intensive Care Med 38, 499–508 (2012). https://doi.org/10.1007/s00134-011-2451-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2451-6