Abstract
Purpose
To evaluate the efficacy of delivering a mixture of helium and oxygen gas (He–O2) in spontaneous ventilation. Three high oxygen flow reservoir masks were tested: the Heliox21, specifically designed for helium; the Hi-Ox80 mask, with an inspiratory and an expiratory valve; and a standard high-concentration face mask.
Methods
This prospective randomized crossover study was performed in six healthy volunteers in a laboratory setting. Volunteers breathed a mixture of 78% He/22% O2 through each of the masks under two different breathing conditions (rest and hyperventilation: minute ventilation of 14.9 ± 6.1 and 26.7 ± 8.7 L min−1, respectively) and four different He–O2 flow rates (7, 10, 12, and 15 L min−1).
Results
A nasopharyngeal catheter was used to estimate He pharyngeal concentration (Fp [He]) in the airways in order to determine the percentage of contamination with room air (% air cont) at end-expiration. Under all testing conditions, the Hi-Ox80 mask presented a significantly lower % air cont. During resting breathing pattern, a Fp [He] higher than 50% was achieved in 54% of the tests performed with the Hi-Ox80 mask compared to 29% for the Heliox21 mask and only 17% for the standard mask. At hyperventilation, a Fp [He] higher than 50% was achieved in 17% of the tests performed with the Hi-Ox mask compared to 4% for the other two masks.
Conclusion
He–O2 administration via the usual high-concentration reservoir masks results in significant dilution by room air. The Hi-Ox80 mask minimized room air contamination and much more frequently achieved a pharyngeal He concentration higher than 50%.
Similar content being viewed by others
Introduction
Helium (He) is an inert gas with a density that is much lower than that of air. As a result, breathing a mixture of helium and oxygen (He–O2) instead of air and oxygen reduces resistance to flow within the airway [1]. Owing to this unique physical property, He–O2 mixtures may potentially be useful in the treatment of conditions such as increased upper airway resistance, asthma, and chronic obstructive pulmonary disease to decrease work of breathing and dyspnea [2–4]. The effects of He–O2 mixtures can also be used for optimizing particle deposition in the human respiratory tract, but will depend on physical properties of the mixture such as density, viscosity, and mean free path [5–7]. The clinical efficacy of breathing an He–O2 mixture, however, has not been firmly established [8].
The success of He-based therapy can depend on the technique and devices used. In ventilated patients, problems can arise from the ventilator performance, whereas in spontaneous ventilation problems come from the mask interface and its leak-prevention capabilities [9]. Indeed, the clinical efficiency of He is directly related to its concentration in the airway. Most clinical studies performed to date have used standard high-concentration reservoir masks that usually provide no more than 70% of oxygen inspired concentration in the upper airway when oxygen is used [10]. Administration of a gas as volatile as He with these types of masks is largely ineffective due to contamination with room air [11]. A study that evaluated helium administration in spontaneous ventilation found a major contamination with ambient air when using a standard face mask; contamination was reduced when an air-cushioned closed anesthetic face mask was used [12]. Nevertheless, prolonged clinical use of this type of mask may be poorly tolerated and more comfortable masks are needed for prolonged administration.
The purpose of the present study was to examine the He–O2 delivery characteristics under various flow rates and breathing patterns of three face masks currently used in spontaneous ventilation. We hypothesized that the He concentration in the patient airway should be at least 50% to provide clinical benefits. On the basis of this clinical boundary condition, we designed and performed a clinical trial to test three He–O2 masks available on the market. One of these masks is advertised as being specifically designed for He administration, the second mask has been designed to provide high oxygen concentrations, and the third is a standard non-rebreathing reservoir mask.
Materials and methods
This was a prospective, randomized, crossover clinical study performed at the Respiratory Research Laboratory of the Medical Intensive Care Unit (ICU) in Henri Mondor Hospital. Signed informed consent in accordance with the criteria established by the hospital ethics committee (Comité de Protection des Personnes) was obtained. Additional information about materials and methods and results are presented in the electronic supplementary material.
Subjects
Evaluations were performed in a randomized order in seven healthy volunteers (3 females and 4 males; age 32 ± 4 years). One volunteer was withdrawn from the study because we were unable to obtain evaluable measurements under any breathing condition. As a result, data from a total of six volunteers were analyzed.
Masks
We tested three masks: the Heliox21® (Intersurgical, UK), the Hi-Ox80 (Viasys, Netherlands), and a standard non-rebreathing reservoir mask (Unomedical, Italy) (Fig. 1).
Helium–oxygen mixture
We used a medicinal He–O2 mixture of 78:22 (Air Liquide Deutschland GmbH), supplied in B10 cylinders, with a pressure reducer and flow meter regulator calibrated according to the gas mixture.
Flows and breathing patterns
Four different flow rates (7, 10, 12, and 15 L min−1) and two breathing patterns (rest and hyperventilation) were evaluated.
Study design
The experimental steps detailed below were performed consecutively for each volunteer in a sitting position during the same half day.
First, all gas analyzer devices were calibrated. A transcutaneous arterial carbon dioxide tension ear-sensor (tcPCO2) (SenTec, Therwil, Switzerland) and two bands of a respiratory inductive plethysmograph (Respitrace Plus™, Non-Invasive Monitoring Systems, US) were placed on the subject. A sampling He catheter connected to a He analyzer (prototype by Viasys, Netherlands) was then inserted through the subject’s nostril into the nasopharynx.
Each subject was first asked to breathe calmly at his/her own resting respiratory rate and tidal volume. Pharyngeal He concentrations [He], plethysmography signals, and tcPCO2 levels were monitored continuously and the data were automatically recorded by the computer.
The first randomly assigned face mask was fitted to the subject and checked to ensure a tight fit to minimize leaks. Once a visual check of the recordings indicated that the subject’s breathing pattern had stabilized, we initiated He–O2 inhalation through the face mask at a delivered flow rate of 7 L min−1. Once [He] had reached a steady state, recordings were continued and stored for 2 min for all parameters. The data from the second minute of these recordings in this stable resting state were used in the final analyses. Next, the subject was asked to increase his/her minute ventilation until tcPCO2 decreased by 6 ± 2 mmHg as compared to the resting tcPCO2 value. After this new hyperventilation stage was considered stable, recordings were continued and stored for 2 min. The face mask under evaluation was then removed and the subject was allowed to breathe ambient room air for several minutes until the [He] reached “0”. The same testing sequences were then repeated using this same face mask to evaluate the various He–O2 flow rates: 10, 12, and 15 L min−1 at rest and in hyperventilation. Then, the same testing protocols were repeated with the other two face masks in the assigned randomized order.
Data collection and processing
[He] signals were digitized and recorded continuously by a data acquisition and analysis program (Acqknowledge, Biopac Systems, USA). Plethysmography signals were recorded independently by a respiratory inductive plethysmograph after quantitative volume calibration performed with a pneumotachometer (Fleish no. 2, Switzerland). This allowed the calculation of minute ventilation for each sequence.
Outcome measures
Because the results of such experiments can be used for any He–O2 mixture, we chose to express the results in terms of percentage of contamination with room air. We used the average of He concentration at end-expiration (Fp [He]) to determine the average percentage of contamination with room air at end-expiration (% air cont) [% air cont = (Fi [He] − mean Fp [He])/Fi [He]]. This % air cont was used as the marker of effectiveness of He delivery.
Statistical analysis
Data are reported as median and ranges. Repeated analysis of variance (ANOVA) measures were used with Tukey’s test, chosen for post hoc comparisons. Statistical significance was assumed when p was less than 0.05.
Results
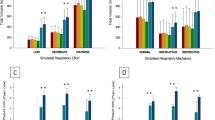
Under resting conditions [average minute ventilation = 15.3 (range 5.6–22.7 L)] and as shown in Fig. 2, the Hi-Ox80 mask presented a significantly lower % air cont at all evaluated flow rates [39% air contamination (range 34–65) at 7 L min−1, 44% (22–59) at 10 L min−1, 37% (17–39) at 12 L min−1, and 25% (11–31) at 15 L min−1] compared with the Heliox21 mask and the standard non-rebreathing reservoir mask [66% (53–70) and 63% (51–75); 53% (41–62) and 54% (43–68); 42% (33–64) and 44% (36–61); 27% (15–57) and 39% (25–52) at 15 L min−1, respectively]. Of the 24 tests performed at rest (4 different flow rates with 6 volunteers), a Fp [He] higher than 50% was achieved in 54% of the tests performed with the Hi-Ox80 mask compared to 29% for the Heliox21 mask and only 17% for the standard mask.
The same differences were observed in hyperventilation (average minute ventilation = 25.4 (range 11.7–38.9 L) where the Hi-Ox80 mask presented a significantly lower % air cont at all tested flow rates [48% air contamination (range 44–80) at 7 L min−1, 51% (30–75) at 10 L min−1, 51% (17–72) at 12 L min−1, and 25% (11–31) at 15 L min−1] compared with the Heliox21 mask and the standard non-rebreathing reservoir mask [78% (71–87) and 74% (68–88); 69% (56–81) and 66% (58–76); 67% (48–76) and 62% (56–82); 57% (24–69) and 52% (38–65) at 15 L min−1, respectively]. A Fp [He] higher than 50% was achieved in 17% of the 24 tests performed with the Hi-Ox mask compared to 4% for the other two masks.
A statistically significant difference between masks applied (p < 0.001), between breathing patterns (p < 0.001), and between delivered flow rates (p < 0.001) was demonstrated. The average % air cont with the Hi-Ox80 was significantly lower than the Heliox21 (adjusted mean difference = −15% [−19 – (−11)% CI], p < 0.001) and the standard mask (adjusted mean difference = −16% [−20 – (−12)% CI], p < 0.001).
Discussion
The present study showed that the effectiveness of He–O2 administration in spontaneous ventilation is poor. For all three masks, room air contamination increased as flow rates decreased and during hyperventilation. However, the Hi-Ox80 mask minimized contamination with ambient air under all conditions assessed.
Clinical implications
The inherent physical properties of He may make it difficult to administer efficiently in spontaneous ventilation, and this has likely been an important problem in numerous trials [13–17]. To achieve the He concentration necessary (>50%) to obtain mechanical advantages, there are essentially three complementary options aside from mechanical ventilation. The first is to use the highest possible concentration of He in the mixture (between 70 and 79%). The second is to use high flows of He–O2, thereby allowing a high inspiratory flow to meet the patient’s requirements for minute volume. The third is to use tight-fitting systems that minimize dilution with ambient air, an approach that is supported by results of a recent laboratory study of healthy volunteers breathing spontaneously [12]. In that study, the authors showed that an air-cushioned anesthetic face mask connected to a disposable anesthetic closed breathing circuit led to the delivery of high He concentrations. The use of such a tight-fitting closed system during spontaneous breathing, however, presents the risk of poor clinical tolerance and increases the risk of developing hypercapnia in predisposed patients. For this reason, its use cannot reasonably be proposed for prolonged periods. In both studies, the administration of He–O2 via a standard high-concentration reservoir mask resulted in significant dilution by room air. A type of mask which can be used in the emergency department or ICU for prolonged periods, e.g., several hours, is therefore needed. Our study demonstrates that a sufficient concentration of He can be provided in the airways under many of the conditions evaluated when appropriate and easy to use interfaces are used with a high proportion of He in the mixture at high flow rates.
Basis for the efficacy of the Hi-Ox80 mask
With its unique system of an inspiratory and expiratory valve that permits the sequential delivery of gas, this mask has already proven to be effective in administering high concentrations of oxygen [18]. Its design allows the initial phase of inspiration—which fills the lower airway—to be performed with the gas (in this case, He–O2) located in the reservoir bag. Then, if the subject’s demand exceeds the flow of He–O2, the inspiratory valve allows air from the exterior to enter and fill the upper airway (anatomical dead space) without diluting the He concentrations in the lower airway.
Limitations
It has to be noted that the tests were carried out without a humidification system. Although no data support the need for airway humidification during such conditions of spontaneous breathing, this may be advisable during prolonged exposure to He–O2 [19]. Which humidification system would be the most effective and the likely impact of humidification on the final [He] are unclear. Water bubble humidification appears to be largely ineffective [20], whereas the use of a heated humidifier could increase the risk of leaks through the different connections.
Conclusion
In the present study we employed a rigorous methodology to evaluate and rationally choose the best mask for He–O2 delivery in spontaneously breathing patients treated in the ICU. The efficacy of He–O2 delivery using commercial face masks is poor. A clinically efficient He concentration can be obtained with high flow rates, but only with certain face masks. This fact may explain the paucity of scientific evidence in terms of the use of He–O2 mixtures during spontaneous breathing. However, our approach resulted in an increase probability of success for future related clinical trials.
References
Hess DR, Fink JB, Venkataraman ST, Kim IK, Myers TR, Tano BD (2006) The history and physics of heliox. Respir Care 51:608–612
Kress JP, Noth I, Gehlbach BK, Barman N, Pohlman AS, Miller A, Morgan S, Hall JB (2002) The utility of albuterol nebulized with heliox during acute asthma exacerbations. Am J Respir Crit Care Med 165:1317–1321
Jaber S, Carlucci A, Boussarsar M, Fodil R, Pigeot J, Maggiore S, Harf A, Isabey D, Brochard L (2001) Helium–oxygen in the postextubation period decreases inspiratory effort. Am J Respir Crit Care Med 164:633–637
Hunt T, Williams MT, Frith P, Schembri D (2010) Heliox, dyspnoea and exercise in COPD. Eur Respir Rev 19:30–38
Peterson JB, Prisk GK, Darquenne C (2008) Aerosol deposition in the human lung periphery is increased by reduced-density gas breathing. J Aerosol Med Pulm Drug Deliv 21:159–168
Apiou-Shirlea G, Simoes-Pichelin M, Caillibotte G, Katz I, Texereau J, Fleming JS, Conway JH, Scheuch G, Martonen TB (2008) Validated three dimensional CFD modelling of aerosol drug deposition in humans—influence of disease and breathing regimens. Respir Drug Deliv 1:185–196
Sandeau J, Katz I, Fodil R, Louis B, Apiou-Sbirlea G, Caillibotte G, Isabey D (2010) CFD simulation of particle deposition in a reconstructed human oral extrathoracic airway for air and helium–oxygen mixtures. J Aerosol Sci 41:281–294
Gainnier M, Forel JM (2006) Clinical review: use of helium–oxygen in critically ill patients. Crit Care 10:241
Fink JB (2006) Opportunities and risks of using heliox in your clinical practice. Respir Care 51:651–660
Sim MA, Dean P, Kinsella J, Black R, Carter R, Hughes M (2008) Performance of oxygen delivery devices when the breathing pattern of respiratory failure is simulated. Anaesthesia 63:938–940
Dhuper S, Choksi S, Selvaraj S, Jha G, Ahmed A, Babbar H, Walia B, Chandra A, Chung V, Shim C (2006) Room air entrainment during beta-agonist delivery with heliox. Chest 130:1063–1071
Standley TD, Smith HL, Brennan LJ, Wilkins IA, Bradley PG, Barrera Groba C, Davey AJ, Menon DK, Wheeler DW (2008) Room air dilution of heliox given by facemask. Intensive Care Med 34:1469–1476
Manthous CA, Hall JB, Caputo MA, Walter J, Klocksieben JM, Schmidt GA, Wood LD (1995) Heliox improves pulsus paradoxus and peak expiratory flow in nonintubated patients with severe asthma. Am J Respir Crit Care Med 151:310–314
Ho AM, Lee A, Karmakar MK, Dion PW, Chung DC, Contardi LH (2003) Heliox vs air-oxygen mixtures for the treatment of patients with acute asthma: a systematic overview. Chest 123:882–890
Rodrigo GJ, Pollack CV, Rodrigo C, Rowe BH (2006) Heliox for non-intubated acute asthma patients. Cochrane Database Syst Rev (4), art no. CD002884. doi:10.1002/14651858.CD002884.pub2
Jaber S, Fodil R, Carlucci A, Boussarsar M, Pigeot J, Lemaire F, Harf A, Lofaso F, Isabey D, Brochard L (2000) Noninvasive ventilation with helium–oxygen in acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 161:1191–1200
Maggiore SM, Richard JC, Abroug F, Diehl JL, Antonelli M, Sauder P, Mancebo J, Ferrer M, Lellouche F, Lecourt L, Beduneau G, Brochard L (2010) A multicenter, randomized trial of noninvasive ventilation with helium–oxygen mixture in exacerbations of chronic obstructive lung disease. Crit Care Med 38:145–151
Slessarev M, Somogyi R, Preiss D, Vesely A, Sasano H, Fisher JA (2006) Efficiency of oxygen administration: sequential gas delivery versus “flow into a cone” methods. Crit Care Med 34:829–834
Shelly MP (2006) The humidification and filtration functions of the airways. Respir Care Clin N Am 12:139–148
Darin J, Broadwell J, MacDonell R (1982) An evaluation of water-vapor output from four brands of unheated, prefilled bubble humidifiers. Respir Care 27:41–50
Acknowledgments
This study has been funded by Air Liquide; EudraCT number, 2007-002521-68. We would like to thank Tom Leenhoven (Cardinal Health, Netherlands) for his kindness in providing logistical support and helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roche-Campo, F., Vignaux, L., Galia, F. et al. Delivery of helium–oxygen mixture during spontaneous breathing: evaluation of three high-concentration face masks. Intensive Care Med 37, 1787–1792 (2011). https://doi.org/10.1007/s00134-011-2355-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2355-5