Abstract
Purpose
To assess sublingual microcirculatory changes following passive leg raising (PLR) and volume expansion (VE) in septic patients.
Methods
This prospective study was conducted in two university hospital intensive care units and included 25 mechanically ventilated patients with severe sepsis or septic shock who were eligible for VE in the first 24 h of their admission. Pulse pressure variation (ΔPP), cardiac output (CO) and sublingual microcirculation indices were assessed at five consecutive steps: (1) semi-recumbent position (Baseline 1), (2) during PLR manoeuvre (PLR), (3) after returning to semi-recumbent position (Baseline 2), (4) at the time when VE induced the same degree of preload responsiveness as PLR (VE∆PP = PLR) and (5) at the end of VE (VEEND). At each step, five sublingual microcirculation sequences were acquired using sidestream darkfield imaging to assess functional capillary density (FCD), microcirculatory flow index (MFI), proportion of perfused vessels (PPV) and flow heterogeneity index (FHI).
Results
The PLR, VE∆PP = PLR and VEEND induced a significant increase in CO and a significant decrease in ΔPP compared to Baseline 1 and Baseline 2 values. Both PLR and VE induced significant increases in FCD, MFI and PPV and a significant decrease in FHI compared to Baseline 1 and Baseline 2 values.
Conclusions
In preload responsive severe septic patients examined within the first 24 h of their admission, both PLR and VE improved sublingual microcirculatory perfusion. At the level of volume infusion used in this study, these changes in sublingual microcirculation were not explained by changes in rheologic factors or changes in arterial pressure.
Similar content being viewed by others
Introduction
Altered microcirculatory blood flow is a major pathophysiological feature of severe sepsis and septic shock [1]. De Backer et al. [2] showed that microvascular density and microvascular blood flow are both reduced in septic patients compared to healthy volunteers or non-septic intensive care unit (ICU) patients. Moreover, the degree of microvascular impairment has a prognostic value since it worsens in non-surviving septic patients compared to those who ultimately overcome their septic episode [3]. Early systemic haemodynamic resuscitation of septic patients may improve the time-course of microcirculatory dysfunction and eventually the patient’s outcome [4, 5]. However, relationships between systemic haemodynamic and microcirculatory changes during resuscitation are complex. In this regard, dobutamine infusion did not induce parallel changes in systemic and sublingual blood flows in septic shock patients [6].
Fluid resuscitation is one of the major therapies aimed at restoring blood pressure and cardiac output (CO) in severe septic patients in the early period as well as in the later phase [7, 8].
Fluid loading may improve microcirculatory blood flow through either systemic effects (such as increased perfusion pressure and/or increased CO), rheologic changes [9] (decreased microvascular blood viscosity) or local vasodilation (shear stress).
The aim of the study reported here was to assess sublingual microcirculatory changes in response to volume expansion (VE) in severe sepsis and septic shock patients eligible for VE within the first 24 h of their admission in the ICU. In order to distinguish between the haemodynamic and rheologic effects of VE on sublingual microvessel perfusion, we also performed passive leg raising (PLR), which is a manoeuvre that mimics VE in terms of preload-related haemodynamic consequences [10, 11] but which is assumed not to exert any rheologic effect. This work has previously been presented in abstract form [12].
Materials and methods
Patients
This observational study was approved by our local Institutional Review Board (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale de Bicêtre), which waived the need for written informed consent. It was conducted in accordance with the ethical standards laid down in the Declaration of Helsinki. Patients were recruited in the surgical and medical ICUs of Bicêtre University Hospital between July 2007 and January 2008. Inclusion criteria were (1) state of severe sepsis or septic shock, as defined by the 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference [13], within the first 24 h of admission to the ICU, (2) preload-dependency, defined by respiratory variations in arterial pulse pressure (ΔPP) greater than 13% [14] and (3) eligibility for VE according to our local guidelines: mean arterial pressure (MAP) <65 mmHg (or a decrease >30 mmHg in previously hypertensive patients), urine output <0.5 mL/kg/h for 2 h and presence of skin mottling. Exclusion criteria were pregnancy, age <18 years, contraindication either to PLR (unstable spine fracture, orthopaedic transtibial leg traction, increased intracranial pressure) or to VE (suspected or confirmed hydrostatic pulmonary oedema), non-sinus rhythm and spontaneous breathing or ventilator triggering. Patients were sedated (midazolam/sufentanil) and mechanically ventilated in volume-controlled mode with a tidal volume ≥7 mL/kg and a 1:2 inspiratory to expiratory ratio.
We recorded the Simplified Acute Physiology Score (SAPS II) [15], the Acute Physiology and Chronic Health Evaluation (APACHE II) score [16] on admission and the Sepsis-related Organ Failure Assessment (SOFA) score [17] at inclusion.
Haemodynamic measurements
The ∆PP was calculated as previously described [14]. As part of routine CO monitoring, patients had either a continuous pulse contour analysis device or an oesophageal Doppler monitor. Details concerning haemodynamic data acquisition are provided in the Electronic Supplementary Material (ESM). At each step of the study, a full set of haemodynamic data was obtained, including heart rate (HR), systolic (SAP), mean (MAP) and diastolic (DAP) arterial pressures, CO, stroke volume (SV) and ∆PP.
Microcirculatory measurements and analysis
Sublingual microcirculation videos were obtained using a side-stream dark field imaging device (SDF; Microscan, MicroVisionMedical, Amsterdam, the Netherlands) derived from the orthogonal polarized spectral imaging technology [18]. Images acquisition and analysis were performed following international recommendations [19] with dedicated software analysis [Automated Vascular Analysis (AVA) ver. 1.0; Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands] as described in the ESM. All sequences were acquired by the same investigator (JP) and then randomly allocated to an alphanumeric code so that neither the patient’s name nor the study step could be identified by a second investigator (SD) that performed the analysis.
Raw quantitative variable assessed with AVA software was functional capillary density (FCD, μm−1 or cm cm−2). As small vessels usually account for more than 90% of sublingual microvasculature and are the most altered in sepsis [2], subsequent analyses were restricted to the small vessel category. Semi-quantitative analysis with AVA provided the microcirculatory flow index (MFI), the proportion of perfused vessels (PPV, %) and the flow heterogeneity index.
Study design
Haemodynamic and microcirculatory indices were assessed at five consecutive steps: (1) semi-recumbent position (Baseline 1), (2) during PLR manoeuvre (PLR), (3) after returning to semi-recumbent position (Baseline 2), (4) at the time when VE induced the same degree of preload responsiveness as PLR (VE∆PP = PLR) and (5) at the end of VE (VEEND) (see ESM and ESM Fig. 1 for details). Volume expansion was performed over 30 min using a maximal volume of either 500 mL normal saline or 500 mL hydroxyethyl starch solution 6% (HES 130/0.4; Voluven, Fresenius Kabi, Sèvres, France) according to the attending physician’s decision. Haemoglobin concentration was measured at the beginning of the study and immediately after VEEND. The ventilator settings, sedative and vasoactive drugs infusion rates were kept constant throughout the study. Patients were followed up for 28-day in-hospital mortality and duration of hospital stay.
Statistical analysis
The distribution of all datasets was checked for normality using the Shapiro–Wilk test and normal chi-square goodness of fit. In the case of non-Gaussian distribution, data were expressed as the median (25th–75th percentiles) and analysed with non-parametric tests: Mann–Whitney test, Spearman correlation ρ, Wilcoxon matched pairs test and Friedman test followed by the Wilcoxon test with Bonferroni correction. When data followed a normal distribution, the results were expressed as mean ± standard deviation (SD) and analysed using paired Student t test and repeated-measures analysis of variance (ANOVA). We checked that there was no association between the type of solution and the changes in the microcirculatory indices. A P value <0.05 was considered to be statistically significant. Data were analysed using StatEl (adScience, Paris, France; http:\\www.adscience.eu) and Prism4 (GraphPad, San Diego, CA) software. A sample size of n = 25 patients was chosen on the basis of feasibility and because for any variable of interest, this sample size allowed a 80% power to detect an effect size (i.e. mean change/standard deviation of change) around 0.65, that was considered as physiologically meaningful, with a alpha risk at 5% adjusted form multiplicity.
Results
Patient characteristics
Twenty-five septic patients (20 with septic shock and 5 with severe sepsis) were included in this study over a 6-month period. Table 1 shows the patient characteristics.
Systemic haemodynamic effects of PLR and VE. No adverse event occurred during the study period. The CO was assessed using a continuous pulse contour analysis device and an oesophageal Doppler monitor in 13 and 12 patients, respectively. The VE was performed with normal saline in eight patients and 6% hydroxyethyl starch solution in 17 patients. The time course of HR, MAP, CO, SV and ΔPP throughout the five sequential study steps is presented in Table 2. The HR remained unchanged throughout the protocol. Compared to Baseline 1, PLR simultaneously induced a significant increase in CO and SV and a significant decrease in ΔPP. No significant difference in any of the haemodynamic indices was found between Baseline 1 and Baseline 2. At the first step of VE (VE∆PP = PLR), MAP, CO and SV were significantly increased compared to both Baseline 1 and Baseline 2 values, and these increases (except for MAP) were not significantly different compared to those at the PLR step. At the end of VE (VEEND), CO and SV increased yet further, and ∆PP continued to decrease. Neither baseline haemodynamics nor VE-induced changes in haemodynamic indices were statistically different between the two subgroups of patients who received either normal saline or hydroxyethyl starch solution, respectively.
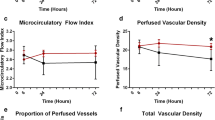
Microcirculatory effects of PLR and VE. We performed 125 SDF studies and therefore recorded 625 video sequences in the 25 septic patients. At Baseline 1, the distribution of microvessels in relation to their diameter was as follows: small vessels, 96.3%; medium vessels, 3.6%; large vessels, 0.03% (data not shown). This distribution was unaltered during the experimental protocol. The time-courses of FCD, MFI, PPV and the flow heterogeneity index during the study period are depicted in Fig. 1a–d, respectively. No significant difference was seen between Baseline 1 and Baseline 2 in terms of the following microvascular indices: FCD, MFI, PPV, and the flow heterogeneity index. The PLR, VE∆PP = PLR and VEEND significantly increased FCD, MFI and PPV compared to the values at Baseline 1 and Baseline 2. The heterogeneity index was significantly reduced by both PLR and VE. Baseline 1 and Baseline 2 values of FCD, MFI, PPV and the heterogeneity index were not significantly different between the patients who received normal saline and those who received hydroxyethyl starch solution. VE-induced changes in microcirculatory indices were also not significantly different between patients receiving normal saline and those receiving the hydroxyethyl starch solution. Haemoglobin values at the beginning of the protocol (Baseline 1: 9.8 ± 1.6 g/dL) and immediately after VE (VEEND: 9.5 ± 1.5 g/dL) were not significantly different for the whole population. However, hydroxyethyl starch-induced VE was associated with a statistically significant decrease in haemoglobin from Baseline 1 (10.3 ± 1.3 g/dL) to VEEND (9.8 ± 1.4, P = 0.007) that was not evidenced in saline-infused patients (P = 0.49). At either baseline or after VE (data not shown), the microcirulatory scores of septic shock patients (n = 20) who received norepinephrine or epinephrine were not significantly different from those of severe sepsis patients (n = 5) without vasoactive drugs. Examples of sublingual microcirculation videos in the same patient at the five steps of the protocol can be seen in the ESM (Animations 1, 2, 3, 4 and 5).
Time-course of sublingual functional capillary density (FCD, cm cm−2) (a), microcirculatory flow index (MFI) (b), proportion of perfused vessels (PPV, %) (c) and flow heterogeneity index (d) throughout the protocol. Baseline 1 patient lying in the 45° semi-recumbent position. PLR Passive leg raising: simultaneous elevation of the lower limbs and lowering of the patient’s trunk to the supine position using automatic bed elevation technique. The hip angle remains constant. PLR induces a reduction in ∆PP (respiratory variations in arterial pulse pressure). Baseline 2 same as Baseline 1. VE ∆PP = PLR , the study step obtained when the volume expansion-induced decrease in ∆PP reaches the PLR-induced ∆PP value. VE END End of volume expansion. The median is shown by the horizontal line within the box. The values between the lower and upper quartiles (25th–75th centiles) are within the box. Whiskers Minimum and maximum values. *P < 0.05 vs. Baseline 1 and Baseline 2; Friedman test followed by the Wilcoxon test with Bonferroni correction
Relationship between microcirculatory perfusion and systemic haemodynamics
There was no statistically significant relationship between PLR-induced changes in macrocirculatory and microcirculatory indices.
At VEEND, VE-induced changes in MFI positively and significantly correlated with VE-induced changes in CO (ρ = 0.53, P < 0.006; ESM Fig. 2a) and MAP (ρ = 0.47, P < 0.018; ESM Fig. 3a). Changes in PPV induced by VE also correlated with VE-induced changes in CO (ρ = 0.51, P < 0.005; ESM Fig. 2b) but not with VE-induced changes in MAP (ρ = 0.29, P = NS; ESM Fig. 3b). No other significant correlation was found between VE-induced changes in microcirculatory indices and macrocirculatory indices.
Discussion
Our study shows that both PLR and VE induced a significant sublingual microcirculatory improvement in preload-dependent patients with severe sepsis and septic shock. Indeed, both PLR and VE simultaneously increased vessel density (increased FCD) and vessel perfusion (increased MFI and PPV) and reduced microvascular heterogeneity. Among the microcirculatory variables investigated, MFI is known to be rather sensitive to flow variations, while FCD and PPV are more directed towards recruitment of the microcirculation. This opposite relationship between changes in MFI or PPV and changes in heterogeneity is in accordance with the results of an experimental study [20] in which animals were bled, thus producing effects in the opposite direction in comparison with those of our study. To the best of our knowledge, these results have not yet been reported in the clinical setting. It has been shown that the application of early goal-directed therapy in septic patients [7] may induce early improvement in sublingual microvascular flow [4] in association with reduced multi-organ failure [5]. Unlike the aforementioned studies, in which microvascular improvement was the result of a global therapeutic approach (including fluid loading, vasopressors, inotropes and blood products), in targeting systemic haemodynamic endpoints, we obtained haemodynamic assessment and SDF images at predefined steps during calibrated manoeuvres of increased preload. The results observed at the different steps of our protocol may enable clinicians to obtain a better understanding of the links between microcirculation and macrocirculation. Indeed, among our patient cohort, microvascular perfusion improvement with VE was not associated with changes in rheologic factors or changes in MAP.
Potential mechanisms of sublingual microcirculatory improvement
The PLR is assumed not to exert any rheologic effect since blood content remains unaltered. A recent study in patients with shock showed that the haemodynamic effects of PLR are only related to increased cardiac preload [21]. Interestingly, in our study, a similar improvement in microcirculatory perfusion was observed after PLR or VE. It is thus unlikely that the changes in microcirculatory perfusion induced by the VE were due to changes in rheologic factors. Based on the results of an experimental study involving severe sepsis patients, Castro et al. [22] reported increases in blood viscosity, decreases in erythrocyte deformability and increases in erythrocyte aggregation in patients receiving hydroxyethyl starch in comparison with those receiving saline. By contrast, we did not observe significant differences in microcirculatory perfusion between patients infused with normal saline and hydroxyethyl starch solution. However, it should be stressed that these conclusions should be limited to the range of changes in rheologic factors associated with the amount of fluid we administered (i.e. 500 mL). In our study, VE-induced changes in MFI were positively correlated with VE-induced changes in MAP (ρ = 0.47) and CO (ρ = 0.53), and VE-induced changes in PPV correlated with VE-induced changes in CO (ρ = 0.51). These results are in agreement with those reported by Trzeciak et al. [4] showing correlations between macrocirculatory and microcirculatory variables in patients studied within 6 h of early goal-directed therapy. However, in our study, MAP did not appear to have a major effect on microcirculation. Indeed, microcirculatory changes were similar during PLR, which was associated with unchanged MAP, and after VE, which was associated with increased MAP up to 7 mmHg on average. Lafanechere et al. [23] also reported such different changes in MAP during PLR and VE in preload-responsive patients. Whether the unchanged MAP during PLR is related to changes in vasomotor tone cannot be excluded, although the hypothesis of altered adrenergic tone during PLR has been refuted by previous investigators [21]. Changes in microcirculatory perfusion were associated with increases in CO induced either by PLR or VE. However, this relationship was not linear since the microcirculatory perfusion remained stable despite the additional increase in CO induced by the second step of the VE (VEEND), suggesting that a threshold was reached. In addition, it should be stressed that the magnitude of the changes in microvascular variables (+94% for MFI, +205% for PPV) was disproportionate compared to that of the changes in CO (+27%). These two points suggest that different mechanisms are implicated in the regulation of microvascular perfusion and in the changes in CO, respectively. Elucidation of the nature of the relation between changes in CO induced by PLR or VE and changes in microcirculatory variables was beyond the scope of our investigation. We may postulate that the increase in CO with PLR and VE further increased microcirculatory perfusion through shear stress-related vasodilation. Neuro-mediated mechanisms interfering with microvascular flow regulation can also be involved. We cannot exclude that changes in sublingual perfusion during PLR were related, at least in part, to putative changes in vasomotor tone. However, and as reported by others [21, 24], the unchanged HR throughout our study makes unlikely the possibility of altered sympathetic tone that could have changed the distribution of blood flow within the macro- or microcirculation.
These data support the hypothesis that VE can improve microcirculatory perfusion during the early period of resuscitation in severe sepsis and septic shock patients. At this stage, this microcirculatory improvement is accompanied by systemic haemodynamic changes, although no causal relationship has yet been established between the regulation of microvascular perfusion and changes in CO, suggesting that different mechanisms are involved.
Limitations of the study
Microvascular analysis was conducted in a blinded fashion and performed with the greatest care in order to avoid pressure artefacts; the most recent published recommendations for such studies were followed [19]. However, the following aspects must be acknowledged: (1) the side-stream dark field imaging device only provides a two-dimensional estimate of a three-dimensional network; (2) the suction device used induced negative pressure, which may have changed the microcirculatory blood flow by interfering with driving pressure. A question which remains to be answered is whether the sublingual mucosa is representative of other areas. A recent experimental study performed by Verdant et al. [25] in pigs with cholangitis support the hypothesis that the sublingual region can indeed be used to monitor the microcirculation in sepsis.
Due to its observational design, our study suffers from a non-standardized VE regimen and a non-standardized measure of CO since both were left to the discretion of the attending physician. However, both the pulse contour analysis device and oesophageal Doppler monitor provide real time CO monitoring and are able to estimate rapid changes in SV [26]. By recruiting only preload-responsive patients, our study design may have favoured some correlation between CO and microcirculatory variables since patients were expected to increase CO after VE. It would have been interesting to also have included patients not predicted to be fluid responders in order to evaluate what would have been their microvascular response. This point should be addressed in future studies. As some of the changes in CO were very large, we cannot exclude the possibility that spontaneous changes in the underlying condition also occurred in those early septic shock patients during the data acquisition period. However, a randomized trial including a group without VE would have been unethical. Another limitation is that we investigated patients at an early period of their disease. Therefore, our results cannot be extrapolated to septic patients receiving VE after the first 24 h of their admission in the ICU.
Conclusions
In preload-responsive patients with severe sepsis and septic shock patients studied during the first 24 h of their ICU stay, both PLR and VE improved sublingual microcirculatory perfusion obtained using side-stream dark field imaging. At the level of VE used in our study, changes in microcirculation were not explained by changes in rheologic factors or changes in MAP. We observed a non-linear relationship between changes in CO and changes in a number of microvascular variables. In addition, the observed changes in microvascular variables were disproportionate compared to changes in CO. These two points suggest that different mechanisms are implicated in the regulation of microvascular perfusion and in the changes in CO.
References
Trzeciak S, Cinel I, Phillip Dellinger R, Shapiro NI, Arnold RC, Parrillo JE, Hollenberg SM (2008) Resuscitating the microcirculation in sepsis: the central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Acad Emerg Med 15:399–413
De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL (2002) Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 166:98–104
Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL (2004) Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 32:1825–1831
Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, Arnold RC, Colilla S, Zanotti S, Hollenberg SM (2007) Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med 49:88–98 e81-82
Trzeciak S, McCoy JV, Phillip Dellinger R, Arnold RC, Rizzuto M, Abate NL, Shapiro NI, Parrillo JE, Hollenberg SM (2008) Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med 34:2210–2217
De Backer D, Creteur J, Dubois MJ, Sakr Y, Koch M, Verdant C, Vincent JL (2006) The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med 34:403–408
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL (2008) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med 34:17–60
Martini J, Cabrales P, Tsai AG, Intaglietta M (2006) Mechanotransduction and the homeostatic significance of maintaining blood viscosity in hypotension, hypertension and haemorrhage. J Intern Med 259:364–372
Maizel J, Airapetian N, Lorne E, Tribouilloy C, Massy Z, Slama M (2007) Diagnosis of central hypovolemia by using passive leg raising. Intensive Care Med 33:1133–1138
Monnet X, Teboul JL (2008) Passive leg raising. Intensive Care Med 34:659–663
Pottecher J, Deruddre S, Georger JF, Vicaut E, Benhamou D, Teboul JL, Duranteau J (2008) Both passive leg raising and volume expansion improve sublingual microcirculation in preload-dependent septic patients. Intensive Care Med 34:S103
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med 29:530–538
Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky MR, Teboul JL (2000) Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med 162:134–138
Le Gall JR, Lemeshow S, Saulnier F (1993) A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Groner W, Winkelman JW, Harris AG, Ince C, Bouma GJ, Messmer K, Nadeau RG (1999) Orthogonal polarization spectral imaging: a new method for study of the microcirculation. Nat Med 5:1209–1212
De Backer D, Hollenberg S, Boerma C, Goedhart P, Buchele G, Ospina-Tascon G, Dobbe I, Ince C (2007) How to evaluate the microcirculation: report of a round table conference. Crit Care 11:R101
Dubin A, Pozo MO, Ferrara G, Murias G, Martins E, Canullan C, Canales HS, Kanoore Edul VS, Estenssoro E, Ince C (2009) Systemic and microcirculatory responses to progressive hemorrhage. Intensive Care Med 35:556–564
Caille V, Jabot J, Belliard G, Charron C, Jardin F, Vieillard-Baron A (2008) Hemodynamic effects of passive leg raising: an echocardiographic study in patients with shock. Intensive Care Med 34:1239–1245
Castro VJ, Astiz ME, Rackow EC (1997) Effect of crystalloid and colloid solutions on blood rheology in sepsis. Shock 8:104–107
Lafanechere A, Pene F, Goulenok C, Delahaye A, Mallet V, Choukroun G, Chiche JD, Mira JP, Cariou A (2006) Changes in aortic blood flow induced by passive leg raising predict fluid responsiveness in critically ill patients. Crit Care 10:R132
Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL (2006) Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med 34:1402–1407
Verdant CL, De Backer D, Bruhn A, Clausi CM, Su F, Wang Z, Rodriguez H, Pries AR, Vincent JL (2009) Evaluation of sublingual and gut mucosal microcirculation in sepsis: a quantitative analysis. Crit Care Med 37:2875–2881
Gunn SR, Kim HK, Harrigan PW, Pinsky MR (2006) Ability of pulse contour and esophageal Doppler to estimate rapid changes in stroke volume. Intensive Care Med 32:1537–1546
Acknowledgments
We are greatly indebted to Mr Nicolas Sandri, computer engineer, for his extensive software knowledge and invaluable technical assistance. Financial supports used for the study only included institutional departmental funds.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: doi:10.1007/s00134-010-1973-7.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Animation 1 (MPG 7608 kb)
Supplementary Animation 2 (MPG 5984 kb)
Supplementary Animation 3 (MPG 7428 kb)
Supplementary Animation 4 (MPG 4122 kb)
Supplementary Animation 5 (MPG 5454 kb)
Rights and permissions
About this article
Cite this article
Pottecher, J., Deruddre, S., Teboul, JL. et al. Both passive leg raising and intravascular volume expansion improve sublingual microcirculatory perfusion in severe sepsis and septic shock patients. Intensive Care Med 36, 1867–1874 (2010). https://doi.org/10.1007/s00134-010-1966-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1966-6