Abstract
Purpose
In acute respiratory distress syndrome (ARDS), combined high-frequency oscillation (HFO) and tracheal gas insufflation (TGI) may improve oxygenation through a TGI-induced increase in mean tracheal pressure (P tr). We compared standard HFO and HFO-TGI matched for P tr, in order to determine whether TGI affects gas exchange independently from P tr.
Methods
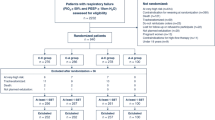
We conducted a prospective, randomized, crossover, physiological study in a 37-bed intensive care unit. Twenty-two patients with early acute lung injury (ALI) or ARDS were enrolled. On day 1, patients were ventilated with HFO, without (60 min) and combined with TGI (60 min) in random order. HFO/HFO-TGI sessions were repeated in inverse order within 7 h. HFO/HFO-TGI mean airway pressure (P aw) was titrated to a P tr that was either equal to (low P aw) or 3 cmH2O higher than (high P aw) the P tr of the preceding conventional mechanical ventilation. On day 2, the protocol was repeated at the alternative P tr level relative to day 1.
Results
Gas exchange and hemodynamics were determined before, during, and after HFO/HFO-TGI sessions. HFO-TGI-high P aw versus HFO-high P aw resulted in significantly higher PaO2/inspired O2 fraction (FiO2) [mean ± standard error of the mean (SEM): 281.6 ± 15.1 versus 199.0 ± 15.0 mmHg; mean increase: 42%; P < 0.001]. HFO-TGI-low P aw, versus HFO-low P aw, resulted in significantly higher PaO2/FiO2 (222.8 ± 14.6 versus 141.3 ± 8.7 mmHg; mean increase: 58%; P < 0.001). PaCO2 was significantly lower during HFO-TGI-high P aw versus HFO-high Paw (45.3 ± 1.6 versus 53.7 ± 1.9 mmHg; mean decrease: 16%; P = 0.037).
Conclusions
At the same P tr level, HFO-TGI results in superior gas exchange compared with HFO.
Similar content being viewed by others
Introduction
In acute respiratory distress syndrome (ARDS), addition of tracheal gas insufflation (TGI) to high-frequency oscillation (HFO) improves gas exchange [1, 2]. TGI may increase carinal pressure and promote lung recruitment [1, 2], improve the washout of the anatomical dead space [2, 3], and augment HFO-dependent, distal gas transport [1–3]. TGI flow of 6.0 L min−1 increases mean tracheal pressure (P tr), even if a cuff leak is used [1]. During standard HFO, P tr increases after a simple clockwise turn of the ventilator’s mean airway pressure (P aw) valve [1]. This may obviate any need for TGI.
We determined the effect of adding TGI to HFO on gas exchange independently from P tr. We compared standard HFO and HFO-TGI matched for a higher and a lower, clinically relevant P tr level [higher P tr level = 3 cmH2O above P tr of preceding conventional mechanical ventilation (CMV); lower P tr level = P tr of preceding CMV; see Electronic Supplementary Material (ESM)]. The rationale for additionally varying P tr was high-pressure-associated recruitment of dependent and well-perfused lung regions. This may improve ventilation–perfusion matching and promote TGI-enhanced distal gas transport [1]. A possible, TGI-related oxygenation improvement [1] could be greater at higher P tr versus lower P tr. Also, a higher P tr could increase physiological dead space, thus altering the effect of TGI on CO2 removal [1]. Consequently, potential differences in gas exchange between HFO and HFO-TGI could depend on P tr level.
Methods
Patients
Institutional approval and informed, written next-of-kin consent were obtained. Patients had early (onset within ≤72 h) acute lung injury or ARDS [4]: PaO2/FiO2 <250 mmHg with positive end-expiratory pressure (PEEP) ≥8 cmH2O for >12 h. Eligibility criteria, sedation, analgesia, neuromuscular blockade, and patient monitoring are detailed in the ESM. Patients received lung-protective CMV; PEEP and FiO2 were set in concordance with the ARDSnet protocol (ESM).
Study design
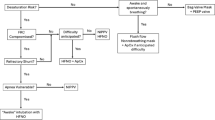
A randomized, crossover design was adopted. Figure 1 illustrates the study protocol. Additional details are provided in the ESM.
Schematic representation of the study protocol. CMV conventional mechanical ventilation, RM recruitment maneuver, HFO high-frequency oscillation, TGI tracheal gas insufflation, P aw mean airway pressure, P tr mean tracheal pressure. * Comprised hemodynamics, gas exchange (arterial and central-venous blood gases), and respiratory mechanics during CMV, and hemodynamics and gas exchange during HFO/HFO-TGI. † Comprised application of continuous positive airway pressure of 45 cmH2O for 40 s. ‡ For day 1, use of a P aw that was either “high” (i.e., titrated to a P tr that was 3 cmH2O higher relative to the P tr of the preceding CMV) or “low” (i.e., titrated to a P tr equal to the P tr of the preceding CMV) was randomized (www.randomizer.org); if day 1 P aw was “high,” day 2 P aw was low, and vice versa; this was done to control for the possible effect of disease evolution on our measurements. § During each day, the order of standard HFO and HFO-TGI was initially randomized and then reversed during the “repeat protocol”; this was done to control for any potential influence of the first HFO/HFO-TGI sessions on gas-exchange results obtained during the immediately subsequent HFO-TGI/HFO sessions
Baseline CMV period
Tracheal tube correct positioning and patency were confirmed, and anesthesia and paralysis were induced. Within 15–20 min post enrollment, blood gases and respiratory compliance were determined. Subsequently, two 4.8-cm-long circuit adapters with side-arms were introduced in between the connector of the tracheal tube and the Y-piece of the breathing circuit (associated equipment dead-space increase = 12.5 mL). A rigid-wall TGI catheter [inner diameter (ID) = 1.0 mm, outer diameter = 2.0 mm] was passed through the proximal adapter. The tip of the TGI catheter reached the tip of the tracheal tube. A second catheter was passed through the distal adapter to monitor P tr. The tip of the pressure-measuring catheter was placed at 2.0 cm beyond the tip of the tracheal tube. The adapter/catheter system was maintained in place during the study intervention periods (Fig. 1). Following recruitment and 55 min of CMV, physiologic measurements were conducted (Fig. 1).
HFO strategies
The Sensormedics 3100B ventilator was connected to the proximal adapter. The TGI catheter was connected to a gas-mixing flowmeter for precise control of the composition of the TGI O2/air mixture. The TGI gas was not humidified, because the use of TGI was intermittent and brief [1]. High-frequency ventilator settings were: FiO2 = FiO2 of the preceding CMV, bias flow = 40 L/min, frequency (f) = 3.5 Hz, pressure amplitude (ΔP) = 90 cmH2O, and inspiratory-to-expiratory time (I:E) ratio = 1:2 (inspiratory time ~0.1 s). On HFO initiation, a recruitment maneuver was performed, a cuff leak of 4–5 cmH2O was placed, and P aw was titrated to the prespecified P tr level (Fig. 1). For HFO-TGI initiation, the recruitment maneuver was repeated, the cuff leak was placed, TGI (FiO2 = FiO2 of preceding CMV; flow = 50% of immediately preceding CMV minute ventilation) was added to HFO, and P aw was titrated to the prespecified P tr level (Fig. 1) [1]. During HFO/HFO-TGI, f and ΔP were to be maintained unchanged unless PaCO2 and pHa could not be kept within ±20 mmHg of the preceding CMV PaCO2 and >7.15, respectively. Within 55–60 min following HFO/HFO-TGI initiation, physiologic measurements were conducted (Fig. 1).
CMV periods following HFO/HFO-TGI
After 120 min of HFO/HFO-TGI, patients were returned to pre-HFO CMV. Within 55–60 min thereafter, physiologic measurements were repeated and the adapters/catheters system was removed (Fig. 1).
Mean esophageal pressure (P es)
In the last five patients, we measured P es as an indicator of lung volume (ESM).
Statistical analysis
According to an a priori power analysis, 22 patients were required for a = 0.048 and power = 0.86. Physiological data were compared using repeated-measures analysis of variance (ANOVA), followed by the Tukey test. Significance was set at P < 0.05. Data are reported as mean ± SEM.
Results
Twenty-two patients were enrolled within a 4-month period (Table 1; ESM). The effect of the catheters on the inspiratory resistance of the tracheal tube (mean increase: 41%) is detailed in the ESM.
Ventilatory variables
Data are presented in Table 2. A lower (by 1–2 cmH2O) P aw was required during HFO-TGI versus standard HFO to achieve the prespecified P tr levels; the corresponding average peak tracheal pressure was also lower (by 2.3–2.7 cmH2O), whereas the average minimal (trough) pressure was 1.3–1.8 cmH2O higher, suggesting a TGI-induced PEEP effect [1, 2]; in five patients, P es was 1.6–4.7 cmH2O higher, indicating a TGI-induced increase in mean lung volume of ~68–139 mL (ESM). P tr was ~4 cmH2O higher during HFO/HFO-TGI-high P aw versus HFO/HFO-TGI-low P aw, because the reference P tr of the preceding CMV (Fig. 1) exhibited a variation of ~1 cmH2O.
Gas exchange
PaO2/FiO2 was higher during HFO-TGI-high P aw versus HFO-high P aw (281.6 ± 15.1 versus 199.0 ± 15.0 mmHg; mean increase: 42%; P < 0.001), and during HFO-TGI-low P aw versus HFO-low P aw (222.8 ± 14.6 versus 141.3 ± 8.7 mmHg; mean increase: 58%; P < 0.001) (Fig. 2). PaCO2 was lower during HFO-TGI-high P aw versus HFO-high P aw (45.3 ± 1.6 versus 53.7 ± 1.9 mmHg; mean decrease: 16%; P = 0.037), and exhibited a trend toward a lower value during HFO-TGI-low P aw versus HFO-low P aw (51.4 ± 1.7 versus 60.0 ± 1.9 mmHg; mean decrease: 14%; P = 0.09). At both P tr levels, pHa was higher during HFO-TGI versus HFO (P < 0.001) (Fig. 2). In five patients, the increase in PaO2/FiO2 and decrease in PaCO2 from HFO to HFO-TGI correlated with the estimated concomitant increase in mean lung volume (r 2 = 0.58–0.71, P ≤ 0.011; ESM, Fig. E5).
Results on gas exchange. FiO 2 inspired O2 fraction, CMV conventional mechanical ventilation, HFO high-frequency oscillation, TGI tracheal gas insufflation, P aw mean airway pressure, CMV pre-/post-high CMV preceding/following HFO-/HFO-TGI-high P aw, CMV pre-/post-low CMV preceding/following HFO-/HFO-TGI-low P aw. Filled squares diamonds, and circles represent mean value, and bars represent standard error of the mean. a Refers to the titration of P aw of HFO/HFO-TGI to either a higher (high P aw) or lower (low P aw) level of mean tracheal pressure (see also “Methods” and Fig. 1). *P < 0.05 versus preceding CMV. † P < 0.05 versus both HFO-high P aw and HFO-TGI-high P aw. § P < 0.05 versus HFO-high P aw. # P < 0.05 versus HFO-TGI-high P aw. ‖ P < 0.05 versus HFO-low P aw. ¶ P < 0.05 versus HFO-TGI-low P aw. **P = 0.09 versus HFO-low P aw
Hemodynamics and CO2 elimination
Ventilatory technique did not affect hemodynamics; shunt fraction was lowest during HFO-TGI-high P aw (Table 3). During CMV and HFO/HFO-TGI, determinants of metabolic rate (e.g., temperature), medication (e.g., vasopressors/inotropes), oxygen consumption, respiratory quotient, cardiac index, and central-venous CO2 concentration remained stable (Table 3; ESM), indicating stable CO2 production and delivery rate to the lungs. Consequently, changes in PaCO2 reflected changes in CO2 elimination efficiency.
Discussion
HFO-TGI versus HFO resulted in higher PaO2/FiO2 (at both P tr levels) and lower PaCO2 (at the higher P tr level). HFO-TGI-induced, proportional blood-gas improvements were comparable between the employed P tr levels. These results imply enhanced lung recruitment and/or gas transport, and alveolar ventilation during HFO-TGI [1, 2, 5].
TGI impedes the opposite-directed expiratory flow, thus exerting a PEEP effect [3]; this explains the higher trough tracheal pressure, despite a lower P aw setting (Table 2). TGI-induced PEEP should increase expiratory lung volume and driving (i.e., alveolar) pressure, with consequent higher expiratory flow and less expiratory airway narrowing or closure [6, 7]. In five patients, the estimated TGI-induced increases in mean lung volume were explanatory of 58–71% of the TGI-induced improvements in gas exchange at both P tr levels. This suggests that lung recruitment is a major mechanism of TGI action. An increase in lung volume augments the axial wall tension of collapsed airways and facilitates their reopening [8], and decreases the diffusional resistance to gas exchange [1].
Preceding imaging studies [9, 10] showed that, at f ≤6 Hz, there is preferential distribution of ventilation in dependent and basal lung regions. Gas streams created by oscillatory flows tend to travel with minimal directional change, favoring lung base filling, since the branching angles of basal airways are less acute [11]. As steady flows behave similarly [12], the addition of TGI to HFO should further enhance lung base aeration, and ventilation–perfusion matching, since the vertical perfusion gradient is maintained during HFO [13]. Basal lung aeration is augmented as HFO tidal volume (V T) and corresponding pressure swings increase [9]. Our f and ΔP settings correspond to V T of ~200 mL [14]. The average, TGI-induced increase in V T was ~11 mL (~5%) (see ESM, Appendix II). TGI may have also acted as an “additional, high-velocity bias flow bypassing the resistance of the tracheal tube and augmenting the effect of a relatively large V T on CO2 elimination” [15].
The calculated mean molecule velocity of the TGI gas at the tip of the TGI catheter was 142.7 m/s, as opposed to an HFO mean inspiratory velocity of ~35.3 m/s at the tip of a tracheal tube with ID of 8.5 mm (ESM, Appendix II). During inspiration, the TGI jet stream may increase the difference between inspiratory and expiratory velocity profiles, and enhance convective gas exchange [16]. The high jet stream velocity implies short dead-space transit time and contribution to direct alveolar ventilation [17]. In the convection–diffusion zones, TGI flow may augment the asymmetry in inspiratory velocity profiles, promote the radial mixing and gas exchange with the expired alveolar gas, and enhance the airway bifurcation phenomenon [17]. Τhe relative importance of the mechanisms of TGI action during HFO warrants further study.
Limitations
Limitations of routine, long-term TGI include absence of commercially available equipment, and possible tracheal mucosal damage, retention and inspissation of secretions, hemodynamic compromise, pneumothorax, and gas embolism [1, 3]. Humidification of TGI gas and cuff leak during HFO-TGI are essential [18]. We excluded patients with 7.5–8.0-mm ID orotracheal tubes, because the two catheters would raise inspiratory tube resistance to >20 cmH2O/L/s (ESM). For long-term TGI, we employ just one catheter, causing an increase in inspiratory tube resistance of <20%, and administer humidified TGI gas [18; ESM].
Clinical implications
When added to CMV, TGI improves CO2 clearance, increases total PEEP, and enables reduction of V T and driving pressures [19, 20]. This may facilitate lung protection in ARDS patients with poor tolerance to hypercapnia due to brain edema, or concurrent metabolic acidosis [19, 20]. In such clinical scenarios, HFO-TGI may prove superior to standard HFO.
Targeting gas-exchange improvements through increases in P aw or V T may accentuate ventilator-associated lung injury. However, HFO-TGI-low P aw versus HFO-high P aw resulted in similar gas exchange (Fig. 2) at lower ventilator-applied lung distending pressure(s), i.e., P aw and P tr (Table 2).
Conclusion
At equal P tr level, HFO-TGI results in superior gas exchange compared with HFO.
References
Mentzelopoulos SD, Roussos C, Koutsoukou A, Sourlas S, Malachias S, Lachana A, Zakynthinos SG (2007) Acute effects of combined high-frequency oscillation and tracheal gas insufflation in severe acute respiratory distress syndrome. Crit Care Med 35:1500–1508
Dolan S, Derdak S, Solomon D, Farmer C, Johanningman J, Gelineau J, Smith RB (1996) Tracheal gas insufflation combined with high-frequency oscillatory ventilation. Crit Care Med 24:456–465
Nahum A (1998) Equipment review: tracheal gas insufflation. Crit Care 2:43–47
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R, the Consensus Committee (1994) The American–European consensus conference on ARDS. Definition, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149:818–824
Ferguson ND, Chiche JD, Kacmarek RM, Hallett DC, Mehta S, Findlay GP, Granton JT, Slutsky AS, Stewart TE (2005) Combining high-frequency oscillatory ventilation and recruitment in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot study. Crit Care Med 33:479–486
Gavriely N, Solway J, Drazen JM, Slutsky AS, Brown R, Loring SH, Ingram RH Jr (1985) Radiographic visualization of airway wall movement during oscillatory flow in dogs. J Appl Physiol 58:645–652
Solway J, Rossing TH, Saari AF, Drazen JM (1986) Expiratory flow limitation and dynamic pulmonary hyperinflation during high-frequency ventilation. J Appl Physiol 60:2071–2078
Gaver DP 3rd, Samsel RW, Solway J (1990) Effects of surface tension and viscosity on airway reopening. J Appl Physiol 69:74–85
Yamada Y, Burnham C, Hales CA, Venegas JG (1989) Regional mapping of gas transport during high-frequency and conventional ventilation. J Appl Physiol 66:1209–1218
Easley RB, Lancaster CT, Fuld MK, Custer JW, Hager DN, Kaczka DW, Simon BA (2009) Total and regional lung volume changes during high-frequency oscillatory ventilation (HFOV) of the normal lung. Respir Physiol Neurobiol 165:54–60
Allen JL, Frantz ID 3rd, Fredberg JL (1985) Regional alveolar pressure during periodic flow. Dual manifestations of gas inertia. J Clin Invest 76:620–629
Slutsky AS, Berdine GG, Drazen JM (1980) Steady flow in a model of human central airways. J Appl Physiol 49:417–423
Rehder K, Didier EP (1984) Gas transport and pulmonary perfusion during high frequency ventilation in humans. J Appl Physiol 57:1231–1237
Hager DN, Fessler HE, Kaczka DW, Shanholtz CB, Fuld MK, Simon BA, Brower RG (2007) Tidal volume delivery during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care Med 35:1522–1529
Khoo MC, Slutsky AS, Drazen JM, Solway J, Gavriely N, Kamm RD (1984) Gas mixing during high-frequency ventilation: an improved model. J Appl Physiol 57:493–506
Scherer PW, Haselton FR (1982) Convective exchange in oscillatory flow through bronchial-tree models. J Appl Physiol 53:1023–1033
Pillow JJ (2005) High-frequency oscillatory ventilation: Mechanisms of gas exchange and lung mechanics. Crit Care Med 33(Suppl):S135–S141
Malachias S, Kokkoris S, Zakynthinos S, Mentzelopoulos SD (2009) High frequency oscillation and tracheal gas insufflation for severe acute respiratory distress syndrome: results from a single-center, phase II, randomized controlled trial [NCT00416260] [abstract]. Intensive Care Med 35(Suppl 1):S6
Richecoeur J, Lu Q, Vieira SRR, Puybasset L, Kalfon P, Coriat P, Rouby JJ (1999) Expiratory washout versus optimization of mechanical ventilation during permissive hypercapnia in patients with severe acute respiratory distress syndrome. Am J Respir Crit Care Med 160:77–85
Martinez-Pérez M, Bernabé F, Peña R, Fernández R, Nahum A, Blanch L (2004) Effects of expiratory tracheal gas insufflation in patients with severe head trauma and acute lung injury. Intensive Care Med 30:2021–2027
Acknowledgements
This work has been funded by the Thorax Foundation, Athens, Greece.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
134_2010_1822_MOESM2_ESM.tif
Experimental setup for in vitro determination of the effect of Vygon catheters on artificial airway resistance. CT, connecting tube. (TIFF 345 kb)
Figure E2.
Pressure drop versus flow relationships for the tracheal tubes employed in the study, and for an 8.0-mm-internal diameter (ID) endotracheal tube. Filled squares denote the presence of two Vygon catheters; empty squares denote the presence of one Vygon catheter; filled diamonds denote the absence of catheter(s). (TIFF 849 kb)
Figure E3.
Results on oxygenation index. FiO2, inspired O2 fraction; CMV, conventional mechanical ventilation; HFO, high-frequency oscillation; TGI, tracheal gas insufflation; Paw, mean airway pressure; CMV pre-/post-high, CMV preceding/following HFO-/HFO-TGI-high Paw; CMV pre-/post-low, CMV preceding/following HFO-/HFO-TGI-low Paw. Empty squares represent mean value, and bars represent standard error of the mean. a Refers to the titration of Paw of HFO/HFO-TGI to either a higher (high Paw) or lower (low Paw) level of mean tracheal pressure (see also “Methods” and Figure 1 of main manuscript). * P < 0.05 versus preceding CMV. § P < 0.05 versus HFO-high Paw. # P < 0.05 versus HFO-TGI-high Paw. ║ P < 0.05 versus HFO-low Paw. (TIFF 160 kb)
Figure E4.
Scan of computer printout of a 30-s part of a representative recording of tracheal and esophageal pressure at the time of the addition of tracheal gas insufflation (TGI) to high-frequency oscillation with low tracheal pressure. At the 5th second, TGI is started at 4 L/min and increased to 7 L/min over the next 5 s. This results in an increase of mean tracheal pressure from 20.1 to 22.8 cmH2O, followed by an increase of mean esophageal pressure from 14.4 to 17.5 cmH2O. At the 15th second, mean airway pressure (Paw) is adjusted, i.e., decreased by 3.0 cmH2O, and mean tracheal pressure returns to its pre-TGI level within the subsequent 5 s. Mean esophageal pressure decreases by 1.4 cmH2O over the next 10 s, and then stabilizes after the 25th second at 16.2 cmH2O, i.e., at 1.8 cmH2O above its pre-TGI level, suggesting a TGI-related increase in lung volume. (TIFF 1.85 mb)
Figure E5.
Relationships between changes in PaO2/inspired O2 fraction (ΔPaO2/FiO2) and PaCO2 (ΔPaCO2) from standard high-frequency oscillation (HFO) to HFO combined with tracheal gas insufflation (TGI) and concomitant changes in mean lung volume (ΔVlung). Data are from the last five study participants (see also “Methods” of main manuscript and “Supplement to Methods”). A, B: Changes from HFO to HFO-TGI with high mean airway and tracheal pressure; C, D: Changes from HFO to HFO-TGI with low mean airway and tracheal pressure. (TIFF 93 kb)
Rights and permissions
About this article
Cite this article
Mentzelopoulos, S.D., Malachias, S., Kokkoris, S. et al. Comparison of high-frequency oscillation and tracheal gas insufflation versus standard high-frequency oscillation at two levels of tracheal pressure. Intensive Care Med 36, 810–816 (2010). https://doi.org/10.1007/s00134-010-1822-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1822-8