Abstract
Mechanical ventilation is a life-saving therapy in several clinical situations, promoting gas exchange and providing rest to the respiratory muscles. However, mechanical ventilation may cause hemodynamic instability and pulmonary structural damage, which is known as ventilator-induced lung injury (VILI). The four main injury mechanisms associated with VILI are as follows: barotrauma/volutrauma caused by overstretching the lung tissues; atelectrauma, caused by repeated opening and closing of the alveoli resulting in shear stress; and biotrauma, the resulting biological response to tissue damage, which leads to lung and multi-organ failure. This narrative review elucidates the mechanisms underlying the pathogenesis, progression, and resolution of VILI and discusses the strategies that can mitigate VILI. Different static variables (peak, plateau, and driving pressures, positive end-expiratory pressure, and tidal volume) and dynamic variables (respiratory rate, airflow amplitude, and inspiratory time fraction) can contribute to VILI. Moreover, the potential for lung injury depends on tissue vulnerability, mechanical power (energy applied per unit of time), and the duration of that exposure. According to the current evidence based on models of acute respiratory distress syndrome and VILI, the following strategies are proposed to provide lung protection: keep the lungs partially collapsed (SaO2 > 88%), avoid opening and closing of collapsed alveoli, and gently ventilate aerated regions while keeping collapsed and consolidated areas at rest. Additional mechanisms, such as subject-ventilator asynchrony, cumulative power, and intensity, as well as the damaging threshold (stress–strain level at which tidal damage is initiated), are under experimental investigation and may enhance the understanding of VILI.
Similar content being viewed by others
Take-home message
Although mechanical ventilation can improve gas exchange and reduce the work of breathing, it may cause ventilator-induced lung injury (VILI). This narrative review elucidates the mechanisms underlying the pathogenesis, progression, and resolution of VILI, and discusses strategies that can mitigate VILI. Different static variables (peak, plateau, driving pressures, positive end-expiratory pressure, and tidal volume) and dynamic variables (respiratory rate, airflow amplitude and profile, and inspiratory time fraction) can contribute to VILI. Additional concepts (mechanical power and subject-ventilator asynchrony) that are currently under investigation are discussed.
According to the current experimental evidence, the following strategies are proposed to provide lung protection: keep the lungs partially collapsed (SaO2 > 88%), avoid opening and closing collapsed alveoli, and gently ventilate aerated regions while keeping collapsed and consolidated areas at rest. In addition, new mechanisms such as cumulative power and intensity, as well as damaging threshold (stress-strain level at which tidal damage is initiated) are under experimental investigation and may enhance the understanding of VILI.
Background
Although mechanical ventilation provides benefits in many clinical situations, it can cause pulmonary structural damage [1], known as ventilator-induced lung injury (VILI), and hemodynamic instability [2]. This is in line with a series of potential harmful effects of mechanical ventilation, including increases in inflammatory infiltration and vascular permeability, hyaline membrane formation, and pulmonary edema. Death may occur during mechanical ventilation even with satisfactory blood gas exchange [3, 4].
The four main injury mechanisms associated with VILI are as follows: barotrauma/volutrauma caused by overstretching the lung tissues; atelectrauma, caused by repeated opening and closing of the alveoli resulting in shear stress; and biotrauma, the resulting biological response to tissue damage, which leads to lung and multi-organ failure [5].
Different static variables (peak, plateau, and driving pressures, positive end-expiratory pressure, and tidal volume) and dynamic variables (respiratory rate [RR], airflow amplitude, and inspiratory time fraction) can contribute to these mechanisms of VILI. Moreover, the potential for lung injury depends on tissue vulnerability, the energy applied per unit of time (mechanical power), and the duration of that exposure [6, 7]. This narrative review discusses the advantages and limitations of experimental VILI, elucidates the mechanisms underlying the pathogenesis, progression, and resolution of VILI, and analyzes the strategies that can mitigate VILI.
Advantages and limitations of experimental VILI
Experimental models allow researchers to investigate the mechanisms of VILI, which would be impossible and/or unethical in humans. Thus, different models of VILI have been developed and studied in diverse animal species in the last decades [8]. Some of the most common VILI models are summarized in Table 1. However, animal studies present some limitations that need to be considered in planning, conducting, and interpreting the results [9]. There are several physiologic and anatomic differences between humans and animals, which may influence the pulmonary response to an acute stimulus [10]. In this context, the RR is higher in mice (250–300 breaths per minute [bpm]) and rats (80–120 bpm) compared with humans (12–16 bpm). In addition, the lung structure of mice does not include bronchial arteries, and the size of the alveolus and the thickness of the alveolar-capillary membrane are smaller than those observed in rats and humans. Unlike the human lung, mice and rats have a monopodial airway branching pattern, whereas the human bronchial tree shows divisions with a dichotomic pattern (each bronchus is divided into two distal bronchi). In terms of inflammatory response, which is important during the development of VILI in animals, mice have lower rates of circulating neutrophils (10–25%) than humans (50–70%) and do not express defensins [11]. The baseline values and the names of neutrophil chemokines differ between rodents and humans, e.g., keratinocyte-derived chemokine in mice versus interleukin-8 in humans. Inter-species differences also exist between humans and pigs and/or piglets. Although the hemodynamics in humans and pigs are similar, the pulmonary vascular response to hypoxia (hypoxic vasoconstriction) is more pronounced in pigs than in humans [12]. To date, no available animal model perfectly mimics all key aspects of human VILI or acute respiratory distress syndrome (ARDS) [8, 13]; nevertheless, current models in use can help us better understand the mechanisms of VILI and develop new therapeutic approaches to mitigate lung damage. Selecting the animal model that most adequately fits the corresponding research question is of utmost importance.
There are additional factors that should be explored further in preclinical studies, such as sex, age, and VILI resolution. Recently, sex was not associated with VILI susceptibility in mice [14]. These findings support the inclusion of both sexes in experimental studies rather than restricting the use of animals of a single sex [15]. Considering that most patients who undergo invasive mechanical ventilation are ~ 60 years old [16], the association between aging organs and mechanical ventilation should be explored further in future preclinical studies. There is insufficient evidence about pulmonary repair mechanisms in experimental VILI. The process after lung injury may involve resolution of alveolar/interstitial edema and inflammation, structural cell proliferation, and extracellular matrix organization [17]. Moreover, modulation of the redox capacity by the Nrf2-ARE pathway has been shown to increase resilience against oxidative stress during injurious mechanical ventilation [18]. In addition, therapy using a conditioned medium obtained from bone marrow and cryopreserved umbilical cord mesenchymal stem cells was able to reduce stretch-induced inflammation and cell death, thus enhancing VILI resolution [19].
Static ventilator variables associated with VILI
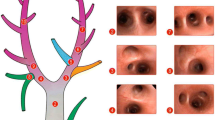
Peak airway pressure (Ppeak,RS), plateau airway pressure (Pplat,RS), positive end-expiratory pressure (PEEP), driving pressure (∆P,RS), and tidal volume (VT) are static ventilator variables associated with VILI (Fig. 1).
Peak airway pressure
In pressure-controlled ventilation (PCV), Ppeak,RS is the maximum pressure during inspiration and depends on the elastic and resistive components (airway, lung tissue) and equipment (endotracheal tube diameter and length) [20, 21]. PCV is usually associated with lower Ppeak,RS compared with volume-controlled ventilation (VCV) due to the different flow profiles, but this difference is less important when the option of ramp flow is used in VCV. In 1974, Webb and Tierney [22] showed that healthy rats ventilated with high Ppeak,RS (45 cmH2O) and zero PEEP presented perivascular and alveolar edema, lung overdistension, and barotrauma. On the other hand, a Ppeak,RS of 45 cmH2O and PEEP of 10 cmH2O did not result in edema. In 2017, Katira et al. [23] reproduced the classic study of Webb and Tierney to clarify these different responses, focusing on heart–lung interaction in healthy rats. They showed that high Ppeak,RS impairs right ventricular filling and pulmonary perfusion, resulting in right ventricular failure and dilation. This scenario is in line with endothelial cell injury and capillary stress failure, which may facilitate microvascular leakage of protein and water into the alveoli, yielding high permeability pulmonary edema. Thus, this preclinical study showed that increased Ppeak,RS values should be avoided due to adverse heart–lung interactions.
Plateau airway pressure
Pplat,RS is calculated during a period when airflow is stopped at end inspiration and reflects end-inspiratory alveolar pressure. Pplat,RS can be affected by changes in VT and respiratory system compliance (C,RS) but not by changes in airflow and airway resistance [24]. The effects of four levels of Pplat,RS (15, 20, 25, and 30 cmH2O) on alveolar-capillary barrier permeability to proteins were studied in a model of lung damage induced by hypertonic solution. Pplat,RS between 20 and 25 cmH2O was associated with epithelial and endothelial cell damage as well as increased permeability [25].
Because Pplat,RS can be affected by the properties of the chest wall, the chest wall component needs to be subtracted from the respiratory system, thus yielding the transpulmonary plateau pressure (Pplat,L) that is associated with the development of VILI. Limiting Pplat,RS to ≤ 28 cmH2O was found to be effective in reducing the risk of overdistension and is widely accepted.
Positive end-expiratory pressure
PEEP reflects the end-expiratory pressure remaining in the airways and, thus, the static preload of the respiratory system. The use of low PEEP levels may not be sufficient to reduce alveolar collapse and lung edema [26]. However, higher PEEP may cause lung overdistention in the more compliant areas of the lungs and hemodynamic impairment. How to best set the PEEP in experimental models of ARDS is still challenging and the following strategies have been described to date: PEEP titrated according to oxygenation, respiratory system compliance or driving pressure, transpulmonary pressure (esophageal pressure), and imaging (computed tomography scan, electrical impedance tomography) [27]. Nevertheless, there are controversies regarding the best PEEP to use in clinical ARDS; it should be set according to each patient considering lung function (arterial blood gases and mechanics), imaging findings (degree of recruitability), and phenotype (hypo- versus hyperinflammatory).
Respiratory system driving pressure
∆P,RS is defined as Pplat,RS-PEEP or VT normalized to C,RS [28], and ΔP,L is defined as the difference between ∆P,L at end inspiration and ∆P,L at end expiration. ∆P,L can be calculated as:
Both ∆P,RS and ΔP,L have been shown to correlate positively with stress and strain [29, 30]. In experimental endotoxin-induced ARDS, different combinations of VT and PEEP were used to create a range of ∆P,L. The combination of a VT of 6 ml/kg and the lowest PEEP and ∆P,L to maintain oxygenation within a normal range minimized VILI even in the presence of alveolar collapse [31]. In agreement with these results, Güldner et al. [32] observed that atelectrauma led to less inflammation than volutrauma strategies (Fig. 2). This strategy of keeping the collapsed lung closed is known as “permissive atelectasis”.
Lung morphology at expiration and inspiration in experimental ARDS, mechanically ventilated with low tidal volume (VT = 6 ml/kg) and progressively increased positive end-expiratory pressure (PEEP). With low VT and low PEEP, aerated lungs (baby lung) are ventilated and collapsed lungs are at rest. With progressive increase in PEEP, at low VT, areas of lung collapse reduce, areas of overdistension increase, and areas of alveolar lung heterogeneity and pendelluft arise; these areas are concentrated around the collapsed units, which present the highest lung stress. At the highest PEEP, the area of lung collapse reduces but even though lung overdistension remains increased, the degree of lung stress and the biological impact on lung tissue reduce because the area associated with pendelluft is no longer observed
Static and dynamic ΔP,L were compared in experimental ARDS. Using the same protective VT, pressure-support ventilation (PSV) resulted in similar static ΔP,L but higher dynamic ΔP,L compared with PCV, leading to higher expression of biomarkers associated with inflammation in PCV [33]. This preclinical study suggested that the main determinant of lung injury is, therefore, the static rather than dynamic ΔP,L.
Tidal volume
Experimental models were also helpful in determining that overdistention rather than inspiratory pressure per se caused lung damage yielding volutrauma. In this context, Dreyfuss et al. [34] reported lung edema in animals ventilated with high VT (40 ml/kg), but such edema did not develop when rats underwent ventilation with increased airway pressures with the use of straps around their abdomens and chests, which reduced the VT (19 ml/kg).
Mechanical ventilation with low VT (4–6 ml/kg) induces repetitive opening and closing of airways and lung units, promoting epithelial cell damage, hyaline membrane formation, and lung edema, which has been named atelectrauma [1]. Interestingly, considering the “baby lung” in ARDS, the shear stress in atelectatic areas induces less lung damage (4–5 times lower) than the force at the edges between aerated and atelectatic lung regions [31, 35].
Recently, Felix et al. [36] showed that in experimental ARDS, lung damage caused by high VT (22 ml/kg) could be attenuated if VT increased slowly enough to progressively (0.5 ml/kg/min) reduce mechanical heterogeneity and allow the epithelial and endothelial cells, as well as the extracellular matrix of the lung, to adapt. In contrast, extending the adaptation period (0.25 ml/kg/min) increased cumulative power and did not prevent lung damage.
Dynamic ventilator variables associated with VILI
The dynamic ventilator variables associated with VILI are the RR, inspiratory airflow, and the inspiratory to expiratory time ratio (Fig. 1).
Respiratory rate
Whereas VT is set to match lung size, RR is usually set to maintain appropriate minute ventilation and meet the patient’s metabolic demand. In contrast to other ventilator variables, RR has been largely neglected compared with other potential variables that cause lung damage. However, when lungs are heterogeneously aerated, as shown in normal lungs [37] and a double-hit VILI model [38], high RR can amplify microstresses and regional strains, thus causing VILI. This phenomenon was shown to be modulated by the degree of pulmonary aeration [39]. The mechanisms of extracellular matrix, epithelial, and endothelial cell adaptation associated with different velocities of increases in RR were recently investigated in rats with experimental ARDS [40]. The animals received abrupt or different gradual increases of RR during protective ventilation. Longer RR adaptation resulted in less lung damage compared with abrupt RR increases. By promoting a gradual increase in RR, alveolar units remain open and better accommodate the stress (reduced airway pressures) for the same strain (VT). On the other hand, by promoting an abrupt increase in RR and shortening inspiratory time, only fast alveolar units remain open, which may favor alveolar overdistension, more heterogeneity, and lung damage. Thus, fast alveolar units, which better accommodate strain, tend to overdistend [41, 42]. After application of the recruitment maneuver, the fraction of slow alveolar units tends to decrease [43], as does the propensity of alveolar units to become atelectatic, which may decrease regional tidal strains and heterogeneity.
Inspiratory airflow
The inspiratory airflow can also be adjusted in some modes of ventilation, which is also a potential cause of VILI [44]. The shear stress at the top of the cells within the respiratory bronchi increased injury. In this context, in situ experiments have shown that healthy lungs support magnitudes of shear stress (15 dyn/cm2) at all alveolar opening velocities in the physiologic range. However, for a lung with increased viscosity of intra-alveoli fluid, shear stress may increase by several orders of magnitude, enough to induce epithelial cell injury [45]. Some reports have associated high inspiratory airflow profiles with gas exchange, the work of breathing, cardiovascular function, and lung damage [46, 47]. Not only the inspiratory airflow amplitude can be harmful but also the airflow waveform (e.g., constant versus decelerating) may be a relatively neglected and modifiable determinant of VILI risk in ARDS [6, 48].
Expiratory airflow: addressing expiration
Traditionally, less attention is given to the expiration phase than to inspiration during controlled mechanical ventilation. Nevertheless, the passive de-pressurization of the respiratory system in conventional ventilation modes predisposes closure of the distal airway and atelectasis formation. However, during so-called flow-controlled ventilation (FCV), airflows during inspiration and expiration are actively controlled and constant, whereas the airway pressure alternates between a peak and end-expiratory pressure, creating a triangular airway pressure profile [49, 50]. Thereby, FCV avoids zero-flow conditions. Along with physiologic improvements, FCV was shown to reduce VILI compared with conventional ventilation [49, 51]. Furthermore, Wittenstein et al. [50] showed that, regardless of fluid status, FCV reduced the mechanical power mainly due to the resistive component compared with VCV during one-lung ventilation. By actively controlling the expiratory phase, the appearance of intrinsic PEEP may be prevented, which in turn promotes better air exhalation among alveoli with different time constants. In a recent preclinical study, Busana et al. [52] studied healthy pigs randomized to a control group and a valve group, where the expiratory flow was controlled through a variable resistor, but all the pigs were ventilated under similar VT, PEEP, and inspiratory airflow. No differences were observed in respiratory mechanics, gas exchange, hemodynamics, wet-to-dry ratios, and histology, whereas the decrease in end-expiratory lung impedance was significantly greater in the control group compared with those that used the variable resistor. The authors concluded that the reduction in expiratory flow occurred mostly across the endotracheal tube and partly in the respiratory system. The beneficial effect of the variable resistor at the expiratory phase may also be dependent on heterogeneous and injured lungs at baseline [53].
Effects of inspiratory to expiratory time ratios
In a model of mild ARDS, mechanical ventilation with increased inspiratory to expiratory ratios (2:1) led to increased gene expression of biological markers associated with inflammation and alveolar epithelial cell damage, whereas a reduced inspiratory to expiratory ratio (1:2) increased markers of endothelial cell damage, and an inspiratory to expiratory of 1:1 minimized lung damage [54]. Similar results were observed in another preclinical study using high VT and prolonged inspiratory time [55].
Mechanical power as a hub for the development of VILI
Mechanical power (MP) is the mechanical energy delivered from the ventilator to the respiratory system and has been considered to be a unifying driver of VILI [49,50,51].
The following formula for MP was described in 2016 [56]:
Not all combinations of the three pressure components of energy (elastic, resistive, and PEEP components) and RR are equally hazardous. Doubling RR increases MP by 1.4-fold, doubling PEEP increases MP by twofold, whereas doubling VT increases MP by fourfold [49]. The increase in transpulmonary MP has been associated with the development of VILI [50]. Moreover, even at low VT, high MP promoted VILI [51]. In short, all combined variables of MP must be considered together [51, 52]. In a study of experimental ARDS in pigs, MP was positively correlated with pulmonary neutrophilic inflammation, which is a mainstay of ARDS pathogenesis [57]. Different formulas for MP have been described [58]. The most simplified version is based on the classic equation of motion:
This formula computes three components, i.e., static PEEP × volume, elastic, and resistive; other formulas compute only the elastic and/or resistive component [59]. Whether the static PEEP × volume component should be included in the MP formula or not is a topic of intense debate [59, 60].
Another point of debate is whether a single measurement of MP in a short time frame (e.g., 1 min) would be clinically meaningful compared with computing the cumulative MP over a relevant time frame, likely better reflecting the time in which subjects are exposed to a certain MP. The cumulative MP has been proposed in preclinical studies [36]. In practical terms, it would be feasible to include the cumulative MP variable at the trends window available in different types of mechanical ventilators. The cumulative MP would reflect (1) all the MP values since the first minute of invasive mechanical ventilation; (2) not only the total amount of MP delivered to patients’ lungs but also how fast the MP is applied; (3) recognition if the injuring strain threshold has been breached [48]; (4) the big panorama of the most injurious ventilator variables, which up to now are not well recognized, such as minute ventilation; (5) the ratio between measured and expected MP [61]. Whether MP, cumulative MP, or MP normalized to lung volume (i.e., intensity) better reflects or predicts VILI is as yet unclear but is currently under investigation in different experimental studies [58, 59].
Asynchronous subject-ventilator interaction as a factor in VILI
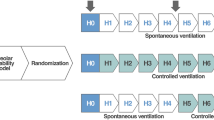
Previously, severe subject-ventilator asynchrony (SVA), i.e., mismatch between the patient respiratory effort and the ventilator support provided, was shown to be associated with worsened clinical outcomes of patients in the intensive care unit and suggested to be causally linked to VILI by increasing transpulmonary pressure and pendelluft [62,63,64]. However, it remained unclear if severe SVA directly contributed to VILI or if it was a symptom or marker rather than a causal factor [65, 66]- This was investigated in a study on mechanically ventilated pigs with experimental ARDS. In that study, SVA (ineffective, auto-, or double-triggering) was actively provoked by random variation of respiratory variables and compared with both assisted and controlled ventilation. It was found that 12 h of severe SVA did not increase lung injury as assessed by histology or by biomarker expression [67], questioning the concept that SVA is directly linked to VILI, at least if lung-protective ventilator settings are respected. However, a different recent study showed contrasting results. In surfactant-depleted rabbits, SVA was induced by phrenic nerve stimulation [68]. Breath stacking was associated with high VT and inspiratory lung stress and yielded both lung and diaphragm injury, and reverse triggering caused diaphragm injury. The discrepancy from the previous study may be explained by different methodological approaches and ventilator settings, especially regarding PEEP. The role of SVA regarding VILI and clinical outcomes warrants further investigation, but the current literature suggests that SVA may not necessarily directly induce VILI.
Future role of experimental studies in intensive care medicine
In the last decades, experimental research has fostered the development of modern intensive care. Experimental studies allow the use of new methods and measurements to effectively investigate lung physiology and pathophysiology. Important mechanisms, such as “lung rest” and the “baby lung concept” [69], have been elucidated based on experimental research, which was then translated to the clinical setting. In contrast, some interventions and concepts that significantly improved physiologic variables in animal studies did not translate into substantially improved clinical outcomes [70]. Currently, there is a tendency toward outcome- and epidemiology-oriented clinical studies with large sample sizes [69]. In addition, social and political movements challenge the need for experimental research. A European Citizen’s Initiative (ECI) recently called for the complete abolition of all animal testing in research in the EU [71], posing an acute and direct risk for animal research in intensive care. After a public hearing, plenary debate in the European Parliament, and cautious analysis, the European Commission recently responded by emphasizing that animal models are currently unavoidable to investigate complex biological or physiologic processes [71].
Accordingly, animal models will continue to play an important role for scientific progress in intensive care, especially because they allow interventions that would not be possible or ethical in humans in potentially life-saving medical interventions. For example, animal models enabled the investigation of actively induced SVA [67] as well as the extensive use of lung imaging techniques in controlled pathophysiologic conditions [72]. Animal studies will continue to allow the necessary translational approach, in which research questions and concepts may be developed in the preclinical setting and transferred to clinical studies to improve intensive care approaches. Research questions and hypotheses will then be derived from the clinical routine and continuously investigated in the experimental setting. For respiratory and mechanical ventilation research, which usually requires an intact cardio-respiratory system, animal experiments may continue to be needed, at least as long as valid alternatives, such as organoids, are not available.
So far, valuable lessons have been learned on the basis of experimental VILI models. However, further open research questions require careful investigation under controlled conditions, which can be optimally achieved with the help of animal models (Table 2). This again emphasizes the future role of experimental research in intensive care medicine.
Conclusion
Preclinical studies using animal models have advanced understanding of the mechanisms of VILI, thus stimulating strategies to mitigate lung damage in patients with ARDS. Even in times of large epidemiological clinical trials, computer modeling studies, and the trend toward abolition of animal testing, innovation, and progress in respiratory and ventilation research, are still based on necessary experimental studies in small and large animals. These studies allow the interpretation of VILI mechanisms and have shown that static and dynamic components are essential variables, which must be controlled by the operator. According to the different models of VILI associated with ARDS: keep the lungs partially collapsed (SaO2 > 88%), avoid opening and closing collapsed alveoli, and gently ventilate aerated regions while keeping collapsed and consolidated areas at rest. Additional mechanisms, such as SVA, cumulative power, intensity, as well as damaging strain threshold (stress–strain level at which tidal damage is initiated), are under experimental investigation and may enhance the understanding of VILI.
Availability of data and materials
Not applicable.
Abbreviations
- ΔP,L :
-
Transpulmonary driving pressure
- ΔP,RS :
-
Driving airway pressure
- ARDS:
-
Acute respiratory distress syndrome
- FCV:
-
Flow-controlled ventilation
- MP:
-
Mechanical power
- PCV:
-
Pressure-controlled ventilation
- PEEP:
-
Positive end-expiratory pressure
- Ppeak,RS :
-
Peak airway pressure
- Pplat,L :
-
Plateau transpulmonary pressure
- Pplat,RS :
-
Plateau airway pressure
- PSV:
-
Pressure-support ventilation
- RR:
-
Respiratory rate
- SVA:
-
Subject-ventilator asynchrony
- VILI:
-
Ventilator-induced lung injury
- VT :
-
Tidal volume
References
Slutsky AS, Ranieri VM (2013) Ventilator-induced lung injury. N Engl J Med 369:2126–2136. https://doi.org/10.1056/NEJMra1208707
Vieillard-Baron A, Matthay M, Teboul JL et al (2016) Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med 42:739–749. https://doi.org/10.1007/s00134-016-4326-3
Avignon PD, Hedenstrom G, Hedman C (1956) Pulmonary complications in respirator patients. Acta Med Scand Suppl 316:86–90. https://doi.org/10.1111/j.0954-6820.1956.tb06263.x
Avignon PD, Lindahl J, Werneman H (1956) Causes of death. Acta Med Scand Suppl 316:111–113
Katira BH (2019) Ventilator-induced lung injury: classic and novel concepts. Respir Care 64:629–637. https://doi.org/10.4187/respcare.07055
Marini JJ, Crooke PS, Gattinoni L (2021) Intra-cycle power: is the flow profile a neglected component of lung protection? Intensive Care Med 47:609–611. https://doi.org/10.1007/s00134-021-06375-5
Caironi P, Langer T, Carlesso E et al (2011) Time to generate ventilator-induced lung injury among mammals with healthy lungs: a unifying hypothesis. Intensive Care Med 37:1913–1920. https://doi.org/10.1007/s00134-011-2388-9
Rocco PRM, Marini JJ (2020) What have we learned from animal models of ventilator-induced lung injury? Intensive Care Med 46:2377–2380. https://doi.org/10.1007/s00134-020-06143-x
Matute-Bello G, Downey G, Moore BB et al (2011) An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 44:725–738. https://doi.org/10.1165/rcmb.2009-0210ST
Kulkarni HS, Lee JS, Bastarache JA et al (2022) Update on the features and measurements of experimental acute lung injury in animals: an official American Thoracic Society workshop report. Am J Respir Cell Mol Biol 66:e1–e14. https://doi.org/10.1165/rcmb.2021-0531ST
Mestas J, Hughes CCW (2004) Of mice and not men: differences between mouse and human immunology. J Immunol 172:2731–2738. https://doi.org/10.4049/jimmunol.172.5.2731
Wolf SJ, Reske AP, Hammermüller S et al (2015) Correlation of lung collapse and gas exchange—a computer tomographic study in sheep and pigs with atelectasis in otherwise normal lungs. PLoS ONE 10:e0135272. https://doi.org/10.1371/journal.pone.0135272
Matute-Bello G, Frevert CW, Martin TR (2008) Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295:L379-399. https://doi.org/10.1152/ajplung.00010.2008
López-Alonso I, Amado-Rodriguez L, López-Martínez C et al (2019) Sex susceptibility to ventilator-induced lung injury. Intensive Care Med Exp 7:7. https://doi.org/10.1186/s40635-019-0222-9
Clayton JA, Collins FS (2014) Policy: NIH to balance sex in cell and animal studies. Nature 509:282–283. https://doi.org/10.1038/509282a
Esteban A, Frutos-Vivar F, Muriel A et al (2013) Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med 188:220–230. https://doi.org/10.1164/rccm.201212-2169OC
González-López A, Albaiceta GM (2012) Repair after acute lung injury: molecular mechanisms and therapeutic opportunities. Crit Care 16:209. https://doi.org/10.1186/cc11224
Veskemaa L, Graw JA, Pickerodt PA et al (2021) Tert-butylhydroquinone augments Nrf2-dependent resilience against oxidative stress and improves survival of ventilator-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol 320:L17–L28. https://doi.org/10.1152/ajplung.00131.2020
Horie S, Gonzalez H, Brady J et al (2021) Fresh and cryopreserved human umbilical-cord-derived mesenchymal stromal cells attenuate injury and enhance resolution and repair following ventilation-induced lung injury. Int J Mol Sci 22:12842. https://doi.org/10.3390/ijms222312842
Bock KR, Silver P, Rom M, Sagy M (2000) Reduction in tracheal lumen due to endotracheal intubation and its calculated clinical significance. Chest 118:468–472. https://doi.org/10.1378/chest.118.2.468
Rocco PR, Zin WA (1995) Modelling the mechanical effects of tracheal tubes in normal subjects. Eur Respir J 8:121–126. https://doi.org/10.1183/09031936.95.08010121
Webb HH, Tierney DF (1974) Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis 110:556–565. https://doi.org/10.1164/arrd.1974.110.5.556
Katira BH, Giesinger RE, Engelberts D et al (2017) Adverse heart–lung interactions in ventilator-induced lung injury. Am J Respir Crit Care Med 196:1411–1421. https://doi.org/10.1164/rccm.201611-2268OC
Silva PL, Rocco PRM (2018) The basics of respiratory mechanics: ventilator-derived parameters. Ann Transl Med 6:376. https://doi.org/10.21037/atm.2018.06.06
de Prost N, Dreyfuss D (1985) Saumon G (2007) Evaluation of two-way protein fluxes across the alveolo-capillary membrane by scintigraphy in rats: effect of lung inflation. J Appl Physiol 102:794–802. https://doi.org/10.1152/japplphysiol.00742.2006
Pássaro CP, Silva PL, Rzezinski AF et al (2009) Pulmonary lesion induced by low and high positive end-expiratory pressure levels during protective ventilation in experimental acute lung injury. Crit Care Med 37:1011–1017. https://doi.org/10.1097/CCM.0b013e3181962d85
Luecke T, Corradi F, Pelosi P (2012) Lung imaging for titration of mechanical ventilation. Curr Opin Anaesthesiol 25:131–140. https://doi.org/10.1097/ACO.0b013e32835003fb
Amato MBP, Meade MO, Slutsky AS et al (2015) Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 372:747–755. https://doi.org/10.1056/NEJMsa1410639
Pistillo N, Fariña O (2018) Driving airway and transpulmonary pressure are correlated to VILI determinants during controlled ventilation. Intensive Care Med 44:674–675. https://doi.org/10.1007/s00134-018-5092-1
Baedorf Kassis E, Loring SH, Talmor D (2016) Mortality and pulmonary mechanics in relation to respiratory system and transpulmonary driving pressures in ARDS. Intensive Care Med 42:1206–1213. https://doi.org/10.1007/s00134-016-4403-7
Samary CS, Santos RS, Santos CL et al (2015) Biological impact of transpulmonary driving pressure in experimental acute respiratory distress syndrome. Anesthesiology 123:423–433. https://doi.org/10.1097/ALN.0000000000000716
Güldner A, Braune A, Ball L et al (2016) Comparative effects of volutrauma and atelectrauma on lung inflammation in experimental acute respiratory distress syndrome. Crit Care Med 44:e854-865. https://doi.org/10.1097/CCM.0000000000001721
Pinto EF, Santos RS, Antunes MA et al (2020) Static and dynamic transpulmonary driving pressures affect lung and diaphragm injury during pressure-controlled versus pressure-support ventilation in experimental mild lung injury in rats. Anesthesiology 132:307–320. https://doi.org/10.1097/ALN.0000000000003060
Dreyfuss D, Soler P, Basset G, Saumon G (1988) High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis 137:1159–1164. https://doi.org/10.1164/ajrccm/137.5.1159
Mead J, Takishima T, Leith D (1970) Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 28:596–608
Felix NS, Samary CS, Cruz FF et al (2019) Gradually increasing tidal volume may mitigate experimental lung injury in rats. Anesthesiology 130:767–777. https://doi.org/10.1097/ALN.0000000000002630
Vaporidi K, Voloudakis G, Priniannakis G et al (2008) Effects of respiratory rate on ventilator-induced lung injury at a constant PaCO2 in a mouse model of normal lung. Crit Care Med 36:1277–1283. https://doi.org/10.1097/CCM.0b013e318169f30e
Retamal J, Borges JB, Bruhn A et al (2016) High respiratory rate is associated with early reduction of lung edema clearance in an experimental model of ARDS. Acta Anaesthesiol Scand 60:79–92. https://doi.org/10.1111/aas.12596
Retamal J, Borges JB, Bruhn A et al (2016) Open lung approach ventilation abolishes the negative effects of respiratory rate in experimental lung injury. Acta Anaesthesiol Scand 60:1131–1141. https://doi.org/10.1111/aas.12735
Xavier PH, Fonseca ACF, Gonçalves LA et al (2023) Lung injury is induced by abrupt increase in respiratory rate but prevented by recruitment maneuver in mild acute respiratory distress syndrome in rats. Anesthesiology 138:420–435. https://doi.org/10.1097/ALN.0000000000004479
Gattinoni L, Marini JJ, Pesenti A et al (2016) The “baby lung” became an adult. Intensive Care Med 42:663–673. https://doi.org/10.1007/s00134-015-4200-8
Hotchkiss JR, Blanch L, Murias G et al (2000) Effects of decreased respiratory frequency on ventilator-induced lung injury. Am J Respir Crit Care Med 161:463–468. https://doi.org/10.1164/ajrccm.161.2.9811008
Del Sorbo L, Tonetti T, Ranieri VM (2019) Alveolar recruitment in acute respiratory distress syndrome: should we open the lung (no matter what) or may accept (part of) the lung closed? Intensive Care Med 45:1436–1439. https://doi.org/10.1007/s00134-019-05734-7
Maeda Y, Fujino Y, Uchiyama A et al (2004) Effects of peak inspiratory flow on development of ventilator-induced lung injury in rabbits. Anesthesiology 101:722–728
Chen Z-L, Song Y-L, Hu Z-Y et al (2015) An estimation of mechanical stress on alveolar walls during repetitive alveolar reopening and closure. J Appl Physiol (1985) 119:190–201. https://doi.org/10.1152/japplphysiol.00112.2015
Smith RA, Venus B (1988) Cardiopulmonary effect of various inspiratory flow profiles during controlled mechanical ventilation in a porcine lung model. Crit Care Med 16:769–772. https://doi.org/10.1097/00003246-198808000-00007
Garcia CSNB, Abreu SC, Soares RML et al (2008) Pulmonary morphofunctional effects of mechanical ventilation with high inspiratory air flow. Crit Care Med 36:232–239. https://doi.org/10.1097/01.CCM.0000295309.69123.AE
Marini JJ, Crooke PS, Tawfik P et al (2021) Intracycle power and ventilation mode as potential contributors to ventilator-induced lung injury. Intensive Care Med Exp 9:55. https://doi.org/10.1186/s40635-021-00420-9
Goebel U, Haberstroh J, Foerster K et al (2014) Flow-controlled expiration: a novel ventilation mode to attenuate experimental porcine lung injury. Br J Anaesth 113:474–483. https://doi.org/10.1093/bja/aeu058
Wittenstein J, Scharffenberg M, Ran X et al (2020) Comparative effects of flow vs. volume-controlled one-lung ventilation on gas exchange and respiratory system mechanics in pigs. Intensive Care Med Exp 8:24. https://doi.org/10.1186/s40635-020-00308-0
Schmidt J, Wenzel C, Spassov S et al (2020) Flow-controlled ventilation attenuates lung injury in a porcine model of acute respiratory distress syndrome: a preclinical randomized controlled study. Crit Care Med 48:e241–e248. https://doi.org/10.1097/CCM.0000000000004209
Busana M, Zinnato C, Romitti F et al (2022) Energy dissipation during expiration and ventilator-induced lung injury: an experimental animal study. J Appl Physiol (1985) 133:1212–1219. https://doi.org/10.1152/japplphysiol.00426.2022
Spraider P, Martini J, Abram J et al (2020) Individualized flow-controlled ventilation compared to best clinical practice pressure-controlled ventilation: a prospective randomized porcine study. Crit Care 24:662. https://doi.org/10.1186/s13054-020-03325-3
Spieth PM, Silva PL, Garcia CSNB et al (2015) Modulation of stress versus time product during mechanical ventilation influences inflammation as well as alveolar epithelial and endothelial response in rats. Anesthesiology 122:106–116. https://doi.org/10.1097/ALN.0000000000000415
Müller-Redetzky HC, Felten M, Hellwig K et al (2015) Increasing the inspiratory time and I:E ratio during mechanical ventilation aggravates ventilator-induced lung injury in mice. Crit Care 19:23. https://doi.org/10.1186/s13054-015-0759-2
Cressoni M, Gotti M, Chiurazzi C et al (2016) Mechanical power and development of ventilator-induced lung injury. Anesthesiology 124:1100–1108. https://doi.org/10.1097/ALN.0000000000001056
Scharffenberg M, Wittenstein J, Ran X et al (2021) Mechanical power correlates with lung inflammation assessed by positron-emission tomography in experimental acute lung injury in pigs. Front Physiol 12:2003. https://doi.org/10.3389/fphys.2021.717266
Giosa L, Busana M, Pasticci I et al (2019) Mechanical power at a glance: a simple surrogate for volume-controlled ventilation. Intensive Care Med Exp 7:61. https://doi.org/10.1186/s40635-019-0276-8
Huhle R, Serpa Neto A, Schultz MJ, Gama de Abreu M (2018) Is mechanical power the final word on ventilator-induced lung injury?—no. Ann Transl Med 6:394. https://doi.org/10.21037/atm.2018.09.65
Vasques F, Duscio E, Pasticci I et al (2018) Is the mechanical power the final word on ventilator-induced lung injury?—we are not sure. Ann Transl Med 6:395. https://doi.org/10.21037/atm.2018.08.17
Gattinoni L, Collino F, Camporota L (2023) Mechanical power: meaning, uses and limitations. Intensive Care Med 49:465–467. https://doi.org/10.1007/s00134-023-06991-3
Thille AW, Rodriguez P, Cabello B et al (2006) Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med 32:1515–1522. https://doi.org/10.1007/s00134-006-0301-8
Blanch L, Villagra A, Sales B et al (2015) Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med 41:633–641. https://doi.org/10.1007/s00134-015-3692-6
Vaporidi K, Babalis D, Chytas A et al (2017) Clusters of ineffective efforts during mechanical ventilation: impact on outcome. Intensive Care Med 43:184–191. https://doi.org/10.1007/s00134-016-4593-z
Sousa de MLA, Magrans R, Hayashi FK et al (2020) Predictors of asynchronies during assisted ventilation and its impact on clinical outcomes: the EPISYNC cohort study. J Crit Care 57:30–35. https://doi.org/10.1016/j.jcrc.2020.01.023
Cronin JN, Formenti F (2023) Experimental asynchrony to study self-inflicted lung injury. Br J Anaesth 130:e44–e46. https://doi.org/10.1016/j.bja.2021.11.020
Wittenstein J, Huhle R, Leiderman M et al (2021) The effect of patient-ventilator asynchrony on lung and diaphragmatic injury in experimental acute respiratory distress syndrome: laboratory study. Br J Anaesth 130:e169–e178. https://doi.org/10.1016/j.bja.2021.10.037
Hashimoto H, Yoshida T, Firstiogusran AMF et al (2023) Asynchrony injures lung and diaphragm in acute respiratory distress syndrome. Crit Care Med. https://doi.org/10.1097/CCM.0000000000005988
Gattinoni L (2023) Curiosity, opportunity, and luck: were the 1970s different? Anesthesiology 139:321–325. https://doi.org/10.1097/ALN.0000000000004626
Pelosi P, Rocco PRM, Gama de Abreu M (2018) Close down the lungs and keep them resting to minimize ventilator-induced lung injury. Crit Care 22:72. https://doi.org/10.1186/s13054-018-1991-3
European Commission (2023) Communication from the Commission on the European Citizens’ Initiative (ECI) ‘Save cruelty-free cosmetics—Commit to a Europe without animal testing. https://single-market-economy.ec.europa.eu/document/download/8718fcbb-cc3b-4695-b48d-b6cfa5e9fc96_en?filename=C_2023_5041_1_EN_ACT_part1_v6.pdf
Bluth T, Kiss T, Kircher M et al (2019) Measurement of relative lung perfusion with electrical impedance and positron emission tomography: an experimental comparative study. Br J Anaesth. https://doi.org/10.1016/j.bja.2019.04.056
Acknowledgements
We would like to thank Moira Elizabeth Shottler, mBA, Rio de Janeiro, Brazil, and Lorna O’Brien (authorserv.com) for editing assistance.
Funding
The Brazilian Council for Scientific and Technological Development (CNPq), Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (Faperj) funded research projects and scholarships.
Author information
Authors and Affiliations
Contributions
PLS, MS, and PRMR wrote the manuscript and revised the final version. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Silva, P.L., Scharffenberg, M. & Rocco, P.R.M. Understanding the mechanisms of ventilator-induced lung injury using animal models. ICMx 11, 82 (2023). https://doi.org/10.1186/s40635-023-00569-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-023-00569-5