Abstract
Objective
To assess the accuracy of a multiplane ultrasound approach to measure pleural effusion volume (PEV), considering pleural effusion (PE) extension along the cephalocaudal axis and PE area.
Methods
Prospective study performed on 58 critically ill patients with 102 PEs. Thoracic drainage was performed in 46 patients (59 PEs) and lung computed tomography (CT) in 24 patients (43 PEs). PE was assessed using bedside lung ultrasound. Adjacent paravertebral intercostal spaces were examined, and ultrasound PEV was calculated by multiplying the paravertebral PE length by its area, measured at half the distance between the apical and caudal limits of the PE.
Results
Ultrasound PEV was compared to either the volume of the drained PE (59 PE) or PEV assessed on lung CT (43 PE). In patients with lung CT, the accuracy of this new method was compared to the accuracy of previous methods proposed for PEV measurement. Ultrasound PEV was tightly correlated with drained PEV (r = 0.84, p < 0.001) and with CT PEV (r = 0.90, p < 0.001). The mean biases between ultrasound and actual volumes of PE were −33 ml when compared to drainage (limits of agreement −292 to +227 ml) and −53 ml when compared to CT (limits of agreement −303 to +198 ml). This new method was more accurate than previous methods to measure PEV.
Conclusion
Using a multiplane approach increases the accuracy of lung ultrasound to measure the volume of large to small pleural effusions in critically ill patients.
Similar content being viewed by others
Introduction
Pleural effusion (PE) is frequently observed in critically ill patients [1]. When abundant, it induces a restrictive syndrome [2] and increases the intrapulmonary shunt by compressing the lung parenchyma [3]. Removal of large PEs by thoracic drainage often improves arterial oxygenation by promoting lung re-aeration [3, 4]. It may also impact the duration of mechanical ventilation favorably [5]. In case of moderate PE, side effects of pleural drainage have to be balanced against limited benefit, with the potential of exposing the patient to an unfavorable benefit/risk ratio [6]. To date, being able to quantify the pleural effusion volume (PEV) accurately at the bedside remains an unresolved problem. The accuracy of bedside frontal chest radiography is poor [7, 8]. Thoracic computed tomography (CT) scans allow an accurate PEV assessment and can be considered as the reference method. Reading of CT sections, however, is time consuming, because manual delineation of PE has to be performed on each CT section in patients with frequent CT attenuations [9]. In addition, performing CT requires transportation of the patient to the Department of Radiology, a risky procedure that cannot be routinely performed in many intensive care units. If repeated, CT results in high radiation exposure for the patient [10, 11]. Recently, ultrasound assessment of PE by measuring its posterior depth has been reported to be correlated with drained PEV [7, 12–14]. For a given PE, however, two factors may reduce posterior depth: thorax dimensions and pulmonary consolidation, which, by preventing lung retraction, contributes to the distribution of pleural fluid along the cephalocaudal axis. In fact, previous ultrasound methods were evaluated in patients with PEs large enough to require thoracic drainage in clinical investigations, and their accuracy could not be assessed for small to large PEs. Moderate and small PEs are precisely the ones for which it is difficult to decide whether or not thoracic drainage should be performed. Quite often, however, drainage of small PEs is indicated for diagnostic reasons instead of therapeutic ones.
The present study was undertaken to assess the validity of a multiplane ultrasound approach taking into account PE extension in the cephalocaudal direction and PE area measured at mid length. The goal was to help the clinical decision making for thoracic drainage by providing an accurate quantification of small to large PEs. The PEV measured by the new method was compared either to the drained PEV or to the PEV measured by CT of the whole lung. In addition, a CT analysis was performed to understand the spatial distribution of PE within the pleural space and to compare the accuracy of different methods previously proposed to assess PEV.
Materials and methods
Patients
For 1 year, critically ill patients whose PEV could be assessed according to a reference technique (either drainage or thoracic CT scan) were prospectively included. Thoracic CT scans or thoracic drainages were performed for clinical reasons related to patient care and distinct from the present study. Exclusion criteria were: loculated PE, fluid leakage during the drainage procedure and incomplete drainage, as assessed by the presence of persisting posterior pleural separation of more than 1 cm using ultrasound 1 h after drainage. The Ethics Committee of La Société de Réanimation de Langue Française approved the study. Because the protocol did not modify routine clinical care, consent for using collected data was later obtained either from patients or their next of kin.
Multiplane ultrasound approach
Bedside pleural ultrasound was performed within 12 h of CT acquisition and within 8 h preceding pleural drainage using a Hitachi-405 (Hitachi Medical Corporation, Tokyo, Japan) with a microconvex 5-MHz probe or a HP Sonos 5500 (Hewlett Packard, Andover, MA) with a 3.5-MHz cardiac phased-array probe. Both investigators who performed the ultrasound pleural assessments were blinded to the CT measurements. The physicians who performed the thoracic drainages were blinded to the ultrasound measurements. The pleural cavity was explored on transverse views in supine patients by positioning the probe in each paravertebral intercostal space (Fig. 1). In order to facilitate PE measurements, the probe was slipped between the patient's back and mattress. The lower and upper intercostal spaces where PE was detected were drawn on the patient’s skin. The PE paravertebral length (L US) was measured between these two points (in cm). Ultrasound PEV (PEVUS) was calculated by multiplying its length (L US) by its cross-sectional area (A US), measured at half the distance between its apical and caudal limits, in a strict transversal plane, at end-expiration. A US was measured as follows: after freezing the image on the screen, the area of PE was manually delineated, and the area was automatically calculated using the ultrasound scanner software (Fig. 2). A US was considered as the mean of three consecutive measurements.
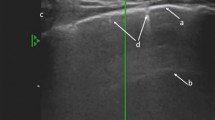
Diagram showing the ultrasound method to quantify PE. The right panel shows a two-dimensional CT reconstruction of the right lung in one of the patients with an extensive bronchopneumonia involving the right lower lobe and associated with a parapneumonic extensive PE. Fluid is located behind the right lower lobe that appears non-aerated, and PE has a double cone shape with a maximal depth at mid thorax. This particular shape is observed in the presence of a consolidated right lower lobe that cannot collapse. In a first step, length was determined by examining each paravertebral intercostal space. PE length (L) was delineated by the thin arrows. The thick arrow shows the intercostal space through which the PE cross-sectional area (A) was measured. The left panel shows the position of the ultrasound probe for measuring cross-sectional PE area at mid PE length. PE is coloured in brown. PE volume was calculated as L × A
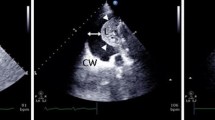
Schematic procedure to quantify PEV with the new formula assessed by ultrasound. The upper (1) and lower (2) paravertebral intercostal spaces where PE is detected are drawn on the patient’s skin. The PE paravertebral length (L US) was measured between these two points (in cm) (3). The middle of the PE height was located (4). At this point, the probe was applied to the skin in a strict transversal plane, at end-expiration, as posterior as possible. The cross-sectional area at mid length (A US) was measured: after freezing the image on the screen, the PE area was manually delineated, and A US was automatically calculated using the ultrasound scanner software (in cm2). A US was considered as the mean of three consecutive measurements. Ultrasound PEV (PEVUS, in ml) was calculated by multiplying L US by A US
Thoracic drainage
An intercostal drain (18–24F) was inserted at the posterior axillary line level after ultrasound location [15]. A negative pressure of −20 cmH2O was applied for active suctioning. One hour later, the amount of PE present in the drainage unit (PEV) was recorded by an independent physician.
Thoracic CT scan
The 1.25- or 2.5-mm-thick contiguous sections of the whole lung were acquired during a prolonged expiratory pause. Injection of contrast material to facilitate the differentiation of PE from consolidated lung parenchyma was left to the decision of the physician in charge of the patient. CT data were stored on computerized disks and subsequently analyzed using Osiris software (version 4.09, University Hospital of Geneva, Switzerland).
For the first step, manual delineation of the PE cross-sectional area was performed on each 5-mm-thick reconstruction by one of the co-authors (ZM), who was blinded to the clinical and ultrasound data. CT PEV (PEVCT) was computed as the total number of pixels present in all PE cross-sectional areas delineated on each transversal CT section times the volume of the voxel [16].
In the second step, additional CT measures were performed by another co-author (FF), who was blinded to the PEVCT, clinical and ultrasound data. CT PE length (L CT) was measured in paravertebral regions between the apical and caudal limits. CT PE depth (PEDCT) was measured in paravertebral regions at the lung base, 2 cm above the diaphragmatic cupola. The largest PE depth between the apical and caudal limits of PE (PEDCTmax) was also measured. PE cross-sectional area (A CT) was measured at the mid length of PE. The largest PE cross-sectional area between apical and caudal limits of PE (A CTmax) was also measured. In nine patients, the spatial distribution of the PE cross-sectional area between the apical and caudal limits of PE was determined (three patients with bilateral PE, three patients with left PE and three patients with right PE).
Study protocol
In a first series of investigations, all available CT scans were analyzed with two objectives: (1) to describe the spatial distribution of PE cross-sectional area between apical and caudal limits of PE, and (2) to compare the accuracy of the new formula PEV = A CT × L CT with the accuracy of previous methods using different formulas validated in CT [17] or in transesophageal echocardiography [18, 19]:
In addition, the accuracy of the mono-dimensional measurement of PEDCT was evaluated.
In a second series of investigations, the multiplane lung ultrasound method using the formula PEVUS = A US × L US was compared to reference methods: PEVCT or drained PEV.
Statistical analysis
Continuous variables were expressed as mean ± SD in case of normally distributed data, or as median (range) in other cases. PEV measured by CT and drained PEV were compared using Student's t test. Correlations were examined by linear regression with 95% prediction and confidence intervals. Bias and limits of agreement were calculated according to Bland-Altman analysis [20]. A p value <0.05 was considered significant. Statistical calculations were performed using Sigmastat Software (SPSS, Inc., San Raphael, CA).
Results
Clinical characteristics of pleural effusions
One hundred two PEs were analyzed in 58 patients (45 males, age 58 ± 17 years, Simplified Acute Physiology Score II 37 ± 14). Main diagnoses of PE were parapneumonic effusions (44%) or capillary leak syndrome due to sepsis (38%). PEV was assessed under mechanical ventilation in 80% of cases and 4–90 days after a thoracotomy in 24% of cases. Mean duration between PEVUS measurement and pleural drainage was 80 min (range 0–930 min) and 202 min (range 107–900 min) between PEVUS measurement and CT. As expected, the mean 59 drained PEVs (in 46 patients) was greater than the mean 43 PEVs assessed on CT in 24 patients (553 ± 205 ml vs. 275 ± 230 ml, p < 0.001). Only 5 of the 43 PEs assessed on CT were drained during the 24 h following the CT scan. Intra-observer variability of PEVUS measurements varied from 8 to 12% depending on the operator. The time necessary to measure PEVUS was less than 10 min.
Computed tomography assessment of PEV
CT distribution of fluid within the pleural cavity
As shown in Figs. 1, 2 and 3, the spatial distribution of PE area between apical and caudal limits of PE had a bi-conical shape made of two cones with a common base, opposite summits, different heights (Figs. 1, 3) and an anterior concavity (Figs. 1, 2). Fluids predominantly accumulated in dependent and paravertebral regions of the pleural space, most often a few centimeters below the mid-length of PE and at a variable distance from the diaphragmatic cupola (Fig. 3). In addition, in the same patient, the right and left sides frequently had different spatial distributions of PE area. Although the spatial distribution of PE areas had a common shape, the wide variability between patients as well as in the same patient between sides precluded any attempt at mathematical modeling. Therefore, the analysis was limited to 12 PEs in 9 patients, and we tried to identify the best and most clinically applicable formula to approach PEV.
Distribution of cross-sectional areas of PE along the cephalocaudal axis in nine critically ill patients. On each contiguous 5-mm-thick computed tomography section, the cross-sectional area of pleural effusion was determined using the Osiris software (version 4.09, University Hospital of Geneva, Switzerland). On the Y axis, each CT section is numbered from the apex to the lung base. On the X axis, the area of pleural effusion is expressed in cm2 from the paravertebral line (continuous vertical line). Three patients had bilateral PE (patients 1, 2 and 3), 3 had a predominant right PE (patients 4, 5 and 6), and 3 had a predominant left PE (patients 8, 9 and 10). The black box indicates the mid length of PE, and the thick horizontal dashed line indicates the position of the diaphragmatic cupola
CT assessment of the accuracy of previous methods of PEV measurement
As shown in Fig. 4, a weak but statistically significant correlation was found between PE juxta-diaphragmatic depth measured at the lung base (PEDCT) [7, 12–14] and PEVCT (r = 0.63, p < 0.001). The large 95% prediction and confidence intervals indicate, however, that measurement of PEDCT alone is not accurate enough to differentiate large from small or moderate PEs.
Correlation between PE paravertebral and juxta-diaphragmatic depth measured at the lung base and PEV measured by CT. R = coefficient of correlation determined by linear regression analysis; curved dotted lines represent the 95% confidence interval, straight dotted lines the 95% prediction interval and the continuous line the linear regression line
As shown in Fig. 5a and online resource 1, a tight and significant correlation (r = 0.95, p < 0.001) was found between PEVCT and calculated PEV using the previously recommended formula: PE length multiplied by maximal PE effusion area (A CTmax × L CT) [18, 19]. The formula, however, systematically overestimated PEVCT, and the wide limits of agreement indicated limited accuracy.
Computed tomography (CT) assessment of accuracy of different formulas used for calculating pleural effusion volume (PEV) in 43 PEs. Figures show linear regression analysis (R = coefficient of correlation, curved dotted lines represent the 95% confidence intervals, straight dotted lines the 95% prediction intervals and continuous line the linear regression line). The corresponding Bland-Altman representations are shown as online resource 1. Figure 5a shows graphics concerning PEV measured on CT (PEVCT) and PEV calculated by the formula: PE length × maximal PE cross-sectional area [18, 19]. Figure 5b shows graphics concerning PEVCT and PEV calculated by the formula: PE length × (maximal paravertebral PE depth)2 [17]. Figure 5c shows graphics concerning PEVCT and the new formula: PE length × PE area at mid-length
As shown in Fig. 5b and online resource 1, a weak but statistically significant correlation (r = 0.71 p < 0.001) was found between PEVCT and calculated PEV using another previously recommended formula: PE length multiplied by maximal PE depth squared (PED 2CTmax × L CT) [17]. The large 95% prediction and confidence intervals and wide limits of agreement suggested limited accuracy.
As shown in Fig. 5c and online resource 1, the formula PE length multiplied by PE cross-sectional area measured at mid PE length (A CT × L CT) was more accurate than previous methods for measuring PEV (r = 0.96, p < 0.001).
Accuracy of multiplane ultrasound for measuring PEV: PEVUS versus PEVCT and PEVUS versus drained PEV
As shown in Fig. 6a and online resource 2, a highly significant correlation was found between PEVUS and PEVCT (r = 0.90, p < 0.001) with a mean bias of −53 ml and limits of agreement ranging between −303 and +198 ml. Similarly, PEVUS was tightly correlated to drained PEV (r = 0.84, p < 0.001) with a mean bias of −33 ml and limits of agreement ranging between −292 and 227 ml (Fig. 6b and online resource 2). Comparison between right (n = 54) and left (n = 48) PEs yielded similar coefficients of correlation between PEVUS and PEVCT (r = 0.85 and 0.94, respectively, p < 0.001) and between PEVUS and drained volume (r = 0.82 and 0.88, respectively, p < 0.001). Neither the presence of mechanical ventilation nor the probe tip shape (microconvex or not) influenced the accuracy of lung ultrasound for PEV measurement (data not shown). When comparing Figs. 6a and 5c, PEV calculated as A US × L US was less accurate than PEV calculated as A CT × L CT, suggesting some limitations of the ultrasound method. The correlation between PEVUS and PEVCT (r = 0.90) was superior to the one between L US and L CT (r = 0.59), suggesting L US could be more difficult to assess than A US.
Accuracy of multiplane lung ultrasound for pleural effusion volume (PEV) measurement using the new formula (PEV = PE length × PE area at mid-length). The reference method is either PEV measured by CT (43 PEs in 24 patients, Fig. 6a) or the drained PEV (59 PEs in 46 patients, Fig. 6b). Figures show linear regression analysis (R = coefficient of correlation, curved dotted lines represent the 95% confidence intervals, straight dotted lines the 95% prediction intervals and continuous line the linear regression line). The corresponding Bland-Altman representations are available in online resource 2
Discussion
Our study demonstrates that the accuracy of bedside lung ultrasound to measure small to large PEs can be improved by using a simple formula (PE length times its area at mid-height). This approach compared favorably with the previous proposed methods, including the measurement of PE depth. Such a result has clinical relevance since the accurate determination of PEV over a wide range of volumes is a critical element in the decision making to perform thoracenthesis.
The first part of the study was aimed at assessing the spatial distribution of PE areas between apical and caudal limits of PE on transversal CT sections, since to our knowledge no data are available on this subject. A more complex form than expected was found, whose dimensions were quite variable from one patient to another and from one side to the other in the same patient (Fig. 3). As a consequence, simple mathematical modeling was impossible. Two different formulas have been previously proposed to estimate PEV: (1) PEV = maximal PE depth squared × PE length [17]; (2) PEV = PE maximal cross-sectional area × PE length [18, 19]. In our study, the PE maximal area level was unpredictable, and because the acoustic window was restricted to intercostals spaces, its localization at bedside with ultrasound may have had poor accuracy (these formulas have been validated in CT or in transesophageal echocardiography). Because PE area at the mid PE length seemed easier to locate, we empirically proposed a new formula for PEV calculation: PEV = PE cross-sectional area at mid-length × PE length. To compare the accuracy of these three methods, CT analyses were preferred to ultrasound measurements. This avoided possible biases due to the technical limitations of ultrasound measurements. The new formula was the most accurate (Fig. 5 and online resource 1). A PEV assessment based exclusively on the basal depth of PE measured 2 cm above the diaphragmatic cupola appears to have poor accuracy for quantifying PEV (Fig. 4). Therefore, it explains why this approach has been described to detect large PEs, not to assess PEV [7, 12–14]. Limitations of the PE depth approach are that no standard protocol of measurement has been described, and that no quantitative definitions of “large” or “moderate” or “small” PEs are available, precluding any clear cutoff for using this measure. One can suspect that the effect of PE on respiratory function will depend not only on its volume, but also on various parameters such as the patient’s height, weight, thoracic and lung morphology. Our results may offer a useful methodological tool for further studies dealing with these questions, allowing bedside PEV assessment without PE drainage.
In the second part of the study, we evaluated the accuracy of a lung ultrasound approach measuring PEV using the best formula identified in the CT study: PEVUS = A US at mid height × L US. PEVUS was tightly correlated with drained PEV in 59 hemithoraces (r = 0.84) and with PEVCT in 43 hemithoraces (r = 0.90, Fig. 6b). When comparing this formula using CT with ultrasound measures, the coefficient of correlation was higher (r = 0.96) and associated with a lower bias and narrower limits of agreement (Figs. 5c, 6a). Such differences suggest some limitations of the ultrasound approach. Measuring PE length and PE area at mid length is less accurate with ultrasound than with CT scans, since acoustic windows are restricted to intercostal spaces. Therefore, physicians may pay particular attention to the following technical aspects of PEVUS assessment. Firstly, the PE length measure seemed to be less accurate than that of PE area: the correlations between CT and ultrasound measures were weaker for PE length than for PEV. Effectively, an error of one intercostal space will induce an important error of about 3 cm in PE length (for a mean PE length of 13.7 cm). Secondly, in case of very large PEs, it is impossible to visualize the entire cross-sectional area on a single ultrasound field, imposing an (possibly erroneous?) extrapolation of surface outlines. Nevertheless, an accurate quantification of very large PEVs is of limited interest, since most physicians will decide to drain it. Thirdly, it is important to obtain ultrasound views as transverse as possible, since oblique deviation tends to overestimate PE area, and so PEV. Fourthly, in contrast to PEVCT, PEVUS does not take into account localized deformations of PE caused by lung consolidations and the amount of fluid present in fissures. Theoretically, these parameters could be analyzed by ultrasound through the (fastidious) assessment of PE cross-sectional area through each intercostal space. Unfortunately, in most patients, the acoustic shadow of the scapula makes the entire visualization of the PE surface in the first intercostal spaces impossible, rendering such overall evaluation impossible. This point explains why our results are no longer valid in the presence of loculated PE due to pleural adhesions that sometimes complicate exudates. Fortunately, these situations seem rare, since one case of four in our study was measured after a thoracotomy. Fifthly, lung ultrasound is observer dependent. Unfortunately, inter-observer variability was not measured in the present study. However, inter-observer variability for PE assessment in patients with acute lung injury and lung re-aeration in patients with ventilator-associated pneumonia has been reported in two recent studies [8, 21]. Kappa values were 0.75 and 0.70, respectively, attesting to a good agreement among observers.
Conclusion
The multiplane approach to PEV, consisting of multiplying the PE length measured in paravertebral regions by the cross-sectional area measured at the mid length, increases the accuracy of bedside lung ultrasound for quantifying PEV in critically ill patients with small, moderate or large PEs. Simple measurement of PE depth at the lung base can be used first as a rapid screening test to detect very large PEs, whereas the multiplane approach can be recommended for assessing small to moderate ones.
Abbreviations
- CT:
-
Computed tomography
- PE:
-
Pleural effusion
- PEV:
-
Pleural effusion volume
- L US :
-
Pleural effusion length measured by ultrasound in paravertebral regions between apical and caudal limits
- A US :
-
Pleural effusion cross-sectional area measured at half the distance between the apical and caudal limits
- PEVUS :
-
Pleural effusion volume measured by ultrasound and calculated as L US × A US
- L CT :
-
Pleural effusion length measured by computed tomography in paravertebral regions between apical and caudal limits
- A CT :
-
Pleural effusion cross-sectional area measured at the mid length of pleural effusion by computed tomography
- A CTMAX :
-
Largest pleural effusion cross-sectional area between apical and caudal limits measured by computed tomography
- PEVCT :
-
Pleural effusion volume measured by computed tomography
- PEDCT :
-
Pleural effusion depth measured at the lung base in paravertebral and juxta diaphragmatic regions by computed tomography
- PEDCTMAX :
-
The largest pleural effusion depth between apical and caudal limits measured by computed tomography
References
Mattison LE, Coppage L, Alderman DF, Herlong JO, Sahn SA (1997) Pleural effusions in the medical ICU: prevalence, causes, and clinical implications. Chest 111:1018–1023
Wang JS, Tseng CH (1995) Changes in pulmonary mechanics and gas exchange after thoracentesis on patients with inversion of a hemidiaphragm secondary to large pleural effusion. Chest 107:1610–1614
Nishida O, Arellano R, Cheng DC, DeMajo W, Kavanagh BP (1999) Gas exchange and hemodynamics in experimental pleural effusion. Crit Care Med 27:583–587
De Waele JJ, Hoste E, Benoit D, Vandewoude K, Delaere S, Berrevoet F, Colardyn F (2003) The effect of tube thoracostomy on oxygenation in ICU patients. J Intensive Care Med 18:100–104
Adenigbagbe A, Kupfer Y, Seneviratne C, Pascal W, Tessler S (2007) Pigtail catheter drainage of transudative pleural effusions hastens liberation from mechanical ventilation (abstract). Chest 132:455S
Remerand F, Luce V, Badachi Y, Lu Q, Bouhemad B, Rouby JJ (2007) Incidence of chest tube malposition in the critically ill: a prospective computed tomography study. Anesthesiology 106:1112–1119
Eibenberger KL, Dock WI, Ammann ME, Dorffner R, Hormann MF, Grabenwoger F (1994) Quantification of pleural effusions: sonography versus radiography. Radiology 191:681–684
Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ (2004) Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 100:9–15
Rouby JJ, Puybasset L, Nieszkowska A, Lu Q (2003) Acute respiratory distress syndrome: lessons from computed tomography of the whole lung. Crit Care Med 31:S285–S295
Brenner DJ, Hall EJ (2007) Computed tomography–an increasing source of radiation exposure. N Engl J Med 357:2277–2284
Brenner DJ (2008) Effective dose: a flawed concept that could and should be replaced. Br J Radiol 81:521–523
Roch A, Bojan M, Michelet P, Romain F, Bregeon F, Papazian L, Auffray JP (2005) Usefulness of ultrasonography in predicting pleural effusions >500 ml in patients receiving mechanical ventilation. Chest 127:224–232
Balik M, Plasil P, Waldauf P, Pazout J, Fric M, Otahal M, Pachl J (2006) Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med 32:318–321
Vignon P, Chastagner C, Berkane V, Chardac E, Francois B, Normand S, Bonnivard M, Clavel M, Pichon N, Preux PM, Maubon A, Gastinne H (2005) Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit Care Med 33:1757–1763
Mayo PH, Goltz HR, Tafreshi M, Doelken P (2004) Safety of ultrasound-guided thoracentesis in patients receiving mechanical ventilation. Chest 125:1059–1062
Puybasset L, Cluzel P, Gusman P, Grenier P, Preteux F, Rouby JJ (2000) Regional distribution of gas and tissue in acute respiratory distress syndrome. I. Consequences for lung morphology. CT Scan ARDS Study Group. Intensive Care Med 26:857–869
Mergo P, Helmberger T, Didovic J, Cernigliaro J, Ros P, Staab E (1999) New formula for quantification of pleural effusions from computed tomography. J Thorac Imaging 14:122–125
Swenson JD, Bull DA (1999) Intraoperative diagnosis and treatment of pleural effusion based on transesophageal echocardiographic findings. Anesth Analg 89:309–310
Capper SJ, Ross JJ, Sandstrom E, Braidley PC, Morgan-Hughes NJ (2007) Transoesophageal echocardiography for the detection and quantification of pleural fluid in cardiac surgical patients. Br J Anaesth 98:442–446
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Bouhemad B, Zhi-Hai L, Arbelot C, Zhang M, Ferrari F, Le-Guen M, Girard M, Lu Q, Rouby JJ (2009) Ultrasound assessment of antibiotic-induced pulmonary re-aeration in ventilator-associated pneumonia. Crit Care Med (in press)
Acknowledgements
The authors are not supported by and do not have any financial interest in any commercial activity that may be associated with the topic of this article.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Remérand, F., Dellamonica, J., Mao, Z. et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med 36, 656–664 (2010). https://doi.org/10.1007/s00134-010-1769-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1769-9