Abstract
Purpose
Endothelial activation has emerged as an early event in the pathogenesis of microcirculatory dysfunction, capillary leakage and multi-organ dysfunction syndrome (MODS). Angiopoietin-2 (Ang-2), a circulating antagonistic ligand of the endothelial-specific Tie2 receptor, has been identified as a non-redundant gatekeeper of endothelial activation. On the basis of our previous report demonstrating release of Ang-2 in endotoxemia and sepsis, we aimed to study the utility of Ang-2 to serve as an outcome-specific biomarker in patients requiring renal replacement therapy (RRT) in the intensive care unit (ICU).
Methods
We measured circulating Ang-2 by ELISA in 117 critically ill patients with AKI at inception of RRT in the ICU. Mortality, length of stay and renal recovery were prospectively assessed during a study period of 28 days after the inception of RRT.
Results
Circulating Ang-2 levels were significantly higher in AKI patients with RIFLE category-Injury or -Failure, compared to patients with RIFLE category-Risk. Elevated levels of circulating Ang-2 correlated with impaired oxygenation, low mean arterial pressure, vasopressor dose and the sequential organ failure assessment (SOFA) score. Ang-2 concentrations were significantly higher in non-survivors than in survivors at day 0 and day 14 after initiation of RRT. Multivariate Cox regression and decision tree analyses confirmed a strong independent prognostic impact of elevated Ang-2 as a predictor of 28-day survival.
Conclusions
The results from this study indicate that circulating Ang-2 is as a strong and independent predictor of mortality in ICU patients with dialysis-dependent AKI.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) requiring renal replacement therapy (RRT) affects approximately 6% of critically ill patients and results in a hospital mortality of 50–60% [1, 2]. Outcome prediction in this selected high-risk collective is challenging due to the lack of appropriate biomarkers and the limited value of severity-of-illness scoring systems [3–6]. Thus, the identification of outcome-specific biomarkers in this patient population would be of great value for ICU physicians.
Recently, the endothelial-specific angiopoietin–Tie ligand-receptor system has emerged as a non-redundant key regulator of endothelial activation [7, 8]. Angiopoietin-1 (Ang-1) and angiopoietin-1 (Ang-2), ligands of the vascular-specific Tie2 receptor [9–11], have gained attention as a novel class of biomarkers in critically ill patients [8]. Constitutive Ang-1/Tie2 signaling maintains vessel integrity, inhibits vascular leakage, suppresses inflammatory gene expression, and prevents recruitment and transmigration of leukocytes [12–14]. In contrast, binding of Ang-2 disrupts protective Ang-1/Tie2 signaling and facilitates endothelial activation and inflammation [7, 15, 16]. In experimental human endotoxemia [17], systemic inflammatory response syndrome (SIRS) and sepsis [18, 19], Ang-2 is rapidly released from endothelial Weibel-Palade bodies (WPB) [20], enters the circulation and becomes accessible for quantification [8].
On the basis of our previous report demonstrating Ang-2 as an independent marker of mortality in medical ICU patients [21], we set out to investigate the ability of serum Ang-2 to serve as an outcome-specific biomarker in high-risk patients with severe AKI requiring RRT in the ICU.
Methods
Patients and study design
The present investigation is a sub-study from the Hannover Dialysis Outcome Trial (HANDOUT), a single-center randomized controlled trial comparing standard and intensified extended dialysis therapy in patients with AKI at seven ICUs of our tertiary care center at the Hannover Medical School between 2003 and 2006. Twenty-nine apparently healthy volunteers [16 males; age 58 (25–73 years)] served as controls.
The protocol and main results of the HANDOUT trial have been published elsewhere [22]. Serum samples for quantification of Ang-2 were available from 117 of 156 patients enrolled (Table 1). All patients underwent extended dialysis using the GENIUS™ dialysis system [23] (Fresenius Medical Care, Bad Homburg, Germany) with high-flux polysulphone dialyzers (F60S, 1.3 m², Fresenius Medical Care, Bad Homburg, Germany).
Inclusion criteria were AKI with RRT dependence indicated by a loss of kidney function of >30% calculated eGFR with either the MDRD or Cockroft–Gault equation or cystatin C GFR within 48 h prior to inclusion and oliguria/anuria (<30 ml/h for >6 h prior to inclusion or hyperkalaemia >6.5 mmol/l) or severe acidosis with pH <7.15. Urine output was determined under optimized conditions (corrected volume status, adequate titration of vasopressors and after an unavailing trial of loop diuretics). Exclusion criteria were pre-existing chronic kidney disease as defined by an MDRD eGFR <50 ml/min or a plasma creatinine concentration >1.7 mg/dl (>150 μmol/l) more than 10 days prior to initiation of the first RRT.
In the current study, AKI at initiation of RRT was classified post-HOC by means of the RIFLE criteria using changes in serum creatinine (see above) and urinary output [24]. Since urinary output was not tracked at 6-h intervals, but only for 24 h, one cannot distinguish between the subgroups of RIFLE-Risk and RIFLE-Injury. Thus, all patients with urinary output <0.5 ml/kg/h were assigned RIFLE-Injury [25].
Physiological parameters, including Sequential Organ Failure Assessment (SOFA) score [26] and Acute Physiology and Chronic Health Evaluation II (APACHE II) score [27], and the presence of sepsis (SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions [28]) were obtained for each patient immediately before initiation of RRT.
Sampling and quantification of angiopoietin-2
Serum samples were obtained immediately before initiation of RRT. Ang-2 was quantified in a blinded fashion by in-house Enzyme Linked Immuno Sorbent Assay as described previously [29].
Outcome definitions
-
Mortality from the day of first dialysis in the ICU (study entry) to death from any cause during a study period of 28 days was the primary outcome studied. Patients who survived to day 28 were censored at day 28.
-
In-hospital mortality was defined as death in the hospital versus the patient being discharged from the hospital alive.
-
Ventilator-free days (VFDs) were defined as the number of days between successful weaning from mechanical ventilation and day 28 after study enrollment. VFDs were 0 if the patient died before day 28 or required mechanical ventilation for ≥28 days.
-
ICU-free days were defined as the number of days between successful transfer to a normal ward and day 28 after study enrollment. ICU-free days were 0 if the patient died before day 28 or stayed in the ICU for 28 days or more.
-
Renal recovery was defined as no need for RRT at day 28 after study enrollment.
Statistical analysis
Continuous variables are expressed as medians with corresponding 25th and 75th percentiles (IQR) and were compared by using the Mann–Whitney rank sum test or the Kruskal–Wallis one-way analysis of variance followed by Dunn’s multiple comparisons test. Categorical variables were compared using the Χ² test. Correlations between variables were assessed by the Pearson correlation coefficient. Standardized mortality ratios with 95% confidence intervals were calculated by dividing the number of observed deaths per group by the mean number of expected deaths per group (based on the APACHE II score) (www.sph.emory.edu/~cdckms/exact-midP-SMR.html). To identify predictors of mortality, Cox’s proportional hazards regression analysis was performed using backward elimination (Wald’s test). Linear regression and binary logistic regression models were used to identify predictors of VFDs, ICU-free days and renal recovery, respectively. To fulfill the assumptions needed for Person correlation and regression analyses, logarithmic (ln) transformation of SOFA score, APACHE II score and Ang-2 was performed. The distribution of the time-to-event variables was estimated using the Kaplan–Meier method with log-rank testing (SPSS package, SPSS Inc., Chicago, IL). Recursive partitioning/decision tree analysis (R package version 3.1-24, www.stats.ox.ac.uk/~ripley/) and receiver–operator characteristic (ROC) curves were used to detect optimal cutoff values for Ang-2 [30]. The validity of the decision tree model was controlled by minimizing the ten-fold cross-validated relative error. All tests were two-sided, and significance was accepted at p < 0.05.
Results
Patient characteristics
Patients were grouped according to RIFLE category-Risk, -Injury or -Failure, respectively (Table 1). Patient groups were comparable with respect to baseline demographics and clinical characteristics, except for the median SOFA score, which was highest in RIFLE-Failure (p = 0.04). Interestingly, the SOFA score without the renal variable was not different between groups (p = 0.3). As expected, oliguria and hyperkalemia were more frequently observed in patients with RIFLE-Failure. RRT was started (i.e., Day 0) after a median (IQR) stay of 2 (1–6) days in the ICU (n = 117). Primary and secondary outcomes were not different between RIFLE category-Risk, -Injury or -Failure, respectively (Table 2).
Ang-2 levels, AKI and MODS at initiation of RRT
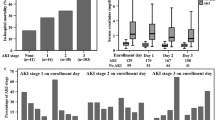
Critically ill patients had significantly higher Ang-2 levels at initiation of RRT than healthy controls [13.5 (6.6–29.4) ng/ml vs. 0.9 (0.8–1.3) ng/ml; p < 0.0001]. Patients with RIFLE category-Risk [4.4 (3.1–6.6) ng/ml] had much lower Ang-2 levels than the category-Injury [15.3 (7.8–36.8) ng/ml; p = 0.009] and the category-Failure [14.4 (8.0–29.4) ng/ml; p = 0.002], respectively. No difference was detected between RIFLE-Injury and -Failure (p = 0.89) (Fig. 1; Table 1). Consistently, there was no linear correlation between Ang-2 levels and serum creatinine (r = 0.14, p = 0.13) or loss of eGFR at initiation of RRT (r = 0.8, p = 0.37).
Correlation of Ang-2 serum levels with AKI. Box and whisker plots showing Ang-2 levels of critically ill patients with AKI at inception of RRT (n = 117) stratified by RIFLE categories Risk (n = 9), Injury (n = 15) and Failure (n = 93). Horizontal bars indicate median values; whiskers indicate 1.5 times the interquartile distance; dots indicate outliers
Ang-2 showed a very weak correlation with unfavorable hemodynamic and pulmonary parameters, such as mean arterial pressure (r = −0.29, p = 0.002), noradrenaline dose (r = 0.36, p < 0.0001) and the alveolar partial oxygen pressure (PaO2)/fraction of inspired oxygen (FiO2) ratio (r = −0.19, p = 0.037). Consistently, Ang-2 concentrations related positively to the SOFA score (r = 0.31, p < 0.001) (Supplementary Material 1). The same was true for the SOFA score without the renal variable (r = 0.28, p = 0.003). However, Ang-2 levels were not related to the APACHE II score (r = 0.18, p = 0.058).
Ang-2 levels did not differ between individual ICUs (Kruskal–Wallis test: p = 0.47) or between patients from medical [16.9 (7.5–36.8) ng/ml] or surgical departments [12.1 (5.9–25.7) ng/ml; p = 0.09], respectively. Ang-2 concentrations were moderately higher in patients with sepsis compared to patients without sepsis [18.8 (10.2–36.8) vs. 11.4 (5.6–26.2) ng/ml); p = 0.012].
Additional data on Ang-2 levels during follow-up and with regard to the effect of RRT are given in Supplementary Material 2.
Predictors of 28-day mortality at inception of RRT
Ang-2 levels were higher in non-survivors [18.8 (12.7–33.9) ng/ml] compared to survivors [9.7 (4.9–22.1) ng/ml; p = 0.0004]. To test whether pre-RRT levels of Ang-2 predict 28-day mortality, we initially performed univariate Cox proportional hazards analyses, incorporating multiple demographic, clinical and laboratory variables (Table 3). All variables found to be statistically significant at a 10% level in the univariate analysis (sepsis/septic shock, SOFA score, APACHE II score, serum creatinine at the start of RRT, and circulating Ang-2) were subjected to multivariate Cox regression analysis. As a result, the presence of sepsis/septic shock (p = 0.038), the SOFA score (p = 0.024) and circulating Ang-2 (p = 0.045) were identified as independent predictors of 28-day mortality. Essentially the same results were obtained in an adjusted model incorporating treatment intensity of RRT (standard vs. intensified extended dialysis in the original HANDOUT trial).
Interestingly, when visualized by Kaplan-Meier curves, 28-day survival was good in the low Ang-2 tertile, but poor in the middle and high tertiles, respectively (log-rank test: p = 0.0003) (Fig. 2). In contrast, mortality steadily increased among SOFA tertiles (p = 0.014), whereas APACHE II tertiles did not allow for identification of clearly distinguishable risk groups (p = 0.118) (Supplementary Material 3).
Secondary outcomes
Consistently, Ang-2 [Odds ratio (OR) 1.63 (95% CI 1.04–2.55); p = 0.033] and the presence of sepsis/septic shock [OR 2.94 (95% CI 1.29–6.72); p = 0.011], but not the SOFA score predicted in-hospital mortality in a multiple binary logistic regression model. Further, Ang-2 predicted the number of VFDs (β 2.26; p = 0.026) in the simple (but not in the multiple) linear logistic regression analysis. In contrast, Ang-2 was not associated with renal recovery at day 28 [OR 1.3 (95% CI 0.77–2.25); p = 0.25] or at hospital discharge [OR 1.5 (95% CI 0.35–6.30)], respectively. The same was true for the number of ICU-free days (β 0.16; p = 0.098), respectively.
Identification of Ang-2 cutoff values and risk stratification by decision tree analysis
Finally, all variables incorporated in the multivariate Cox model were concurrently subjected to regression tree analysis to recursively identify best predictors and patient subgroups with different prognosis for 28-day mortality. Most notably, the optimal recursively identified variable in this model was an Ang-2 cutoff value of 8.78 ng/ml (close to the lower Ang-2 tertile of <9.2 ng/ml). Consistently, the Ang-2 cutoff value of 8.78 ng/ml proved to be statistically significant when tested by multivariate Cox regression (p < 0.001) (Table 3) or log-rank test (p < 0.0001), respectively (Supplementary Material 3).
When visualized by a ROC procedure, the area under the curve (AUC) was 0.70 ± 0.05 (95% CI 0.60–0.79; p < 0.001) (Fig. 3a). An Ang-2 value of ≥8.78 ng/ml predicted death with a sensitivity of 93% (95% CI 84–98) and a specificity of 49% (95% CI 43–51) (chi-square test: p < 0.0001). The positive predictive value for 28-day mortality was 53%. In contrast, a serum Ang-2 < 8.78 ng/ml had a negative predictive value for mortality of 92%. The 28-day mortality of patients among the high Ang-2 group was 53.8%, whereas it was only 10.3% in the low Ang-2 group (Fig. 3b).
Identification of Ang-2 cutoff values and risk stratification by decision tree analysis. a Receiver-operator characteristic (ROC) curve showing the prognostic sensitivity and specificity of circulating Ang-2 at initiation of RRT with regard to 28-day mortality [AUC: 0.70 ± 0.05 (95% CI 0.60–0.79); p < 0.001]. A cuboid indicates the recursively identified Ang-2 cutoff value in this model (8.78 ng/ml). This Ang-2 cutoff value of 8.78 ng/ml predicted 28-day mortality with a sensitivity of 93% (95% CI 84–98) and a specificity of 49% (95% CI 43–51), respectively (chi-square test: p < 0.0001). b A recursively identified Ang-2 cutoff value of 8.78 ng/ml (at the inception of renal replacement therapy) segregates critically ill patients with acute kidney injury into two significantly different risk groups for 28-day mortality (p < 0.0001 by chi-square test) (CI confidence interval). The positive predictive value for 28-day mortality was 53%, whereas the predictive value for mortality was 92%
Discussion
The present clinical investigation is a validation study on the prognostic utility of circulating Ang-2 as an outcome-specific biomarker in a high-risk cohort of critically ill patients with AKI treated with RRT. Ang-2 was identified as a valuable predictor of 28-day and in-hospital mortality, respectively.
The ability to measure Ang-2 in human serum and plasma samples has provided the foundation for investigations centered on Ang-2 as a biomarker in intensive care medicine [8, 29]. Early experimental and recent clinical studies suggested that Ang-2 might constitute a specific marker/mediator of pulmonary vascular permeability and injury, leading to acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) [19, 31–33]. In accordance with previous studies, we observed a very weak albeit significant association between circulating Ang-2 and pulmonary dysfunction (PaO2/FiO2 index). Moreover, Ang-2 predicted VFDs in our cohort.
However, it is likely that the pulmonary and systemic vascular endothelium is simultaneously activated, since both are exposed to circulating inflammatory mediators. This hypothesis is reinforced by several aspects of the present study: First, we observed a significant association between elevated Ang-2 levels with inadequate MAP and hyporesponsiveness to vasopressors. Although this observation does not prove causality, it is in line with a close correlation between circulating Ang-2 and the heart rate/mean arterial pressure index as a surrogate marker for hemodynamic compromise during human endotoxemia [17]. Second, circulating Ang-2 levels were significantly higher in patients with the RIFLE categories Injury and Failure compared to patients with the RIFLE category Risk. Third, consistent with previous reports [18, 21, 29], Ang-2 levels correlated with the SOFA score (with or without the renal variable), suggesting an association of Ang-2 with the extent of MODS. It is thus conceivable to assume that Ang-2 is not an exclusive marker of ALI/ARDS (or AKI), but that elevated Ang-2 actually reflects excessive endothelial activation and barrier dysfunction throughout the entire vascular tree.
It is not clear whether elevated circulating Ang-2 is just a marker of endothelial activation or if it actively contributes to endothelial pathology in humans. At least in pre-clinical models, there is compelling evidence for a mediator function of (circulating) Ang-2 [19, 34]. Intriguingly, the renal vasculature might be a rich source of Ang-2. There is preclinical evidence that AKI causes a rise in systemic levels of circulating Ang-2 and vWF (co-stored in WPB) [35, 36]. In addition, elevated Ang-2 levels in patients with active ANCA-associated vasculitis [37] and systemic lupus erythematosus [38] have been linked to renal involvement. However, the absence of Ang-2 (~55 kDa as a monomer) from the urine of apparently healthy persons almost excludes glomerular filtration or tubular secretion as physiological routes of Ang-2 clearance from the circulation [37]. In line with this, extended dialysis did not affect circulating Ang-2 levels in the present study.
As a prepackaged constituent of WPB, it is not surprising that Ang-2 levels on ICU admission are increased in response to early endothelial activation in critically ill patients [21, 32, 39–42]. However, the predictive utility of Ang-2 throughout the course of critically illness is poorly characterized and has not been tested specifically in AKI patients at the initiation of RRT. As in our previous study on Ang-2 admission levels in medical ICU patients [21], we could identify elevated Ang-2 (measured at initiation of RRT) as an independent predictor of 28-day mortality in a multivariate Cox model. Moreover, non-survivors not only presented with higher Ang-2 levels at initiation of RRT, but also showed elevated Ang-2 levels at day 14 compared to survivors, respectively. Interestingly, we recently detected a very similar predictive Ang-2 pattern during a 72-h time course in septic patients [17].
In addition to the classical Cox’s regression approach [30], recursive partitioning identified a distinctive Ang-2 cutoff value (8.78 ng/ml) that best segregates survivors from non-survivors. However, due to a rather poor positive predictive value of only 53%, a serum Ang-2 level of ≥8.78 ng/ml does not serve as a robust biomarker for predicting mortality, at least in this cohort of patients. Importantly, however, a serum Ang-2 level <8.78 ng/ml may have the potential to predict survival with a negative predictive value of 92%. In other words, the observed mortality was only 10.3% in patients with an Ang-2 level <8.78 ng/ml, despite a predicted average hospital mortality of 55% according to the APACHE II score. These data indicate an important limitation for Ang-2 as an outcome-specific biomarker: low Ang-2 is an excellent indicator of survival, but elevated Ang-2 is not necessarily associated with high mortality in an individual patient.
Several limitations of the current study deserve discussion. First, the robustness of RIFLE for outcome prediction has been demonstrated by several large observational studies [25, 43]. However, RIFLE did not predict mortality or secondary outcomes in the current study. Second, standardized mortality ratios at either ICU admission or initiation of RRT were much lower than expected from severity of disease scorings and did not increase with RIFLE classes. Both findings may be explained by substantial differences in study design. Of note, our study population consisted exclusively of patients requiring RRT. Hence, the robustness of RIFLE is hampered by low numbers of subjects with RIFLE-Risk and -Injury, and the absence of non-AKI patients. In addition, patients were classified by RIFLE at initiation of RRT, whereas most investigators have used other time points (e.g., worst RIFLE during the study period) [43]. However, our results are in accordance with a study by Maccariello et al. [5], who reported that RIFLE was not associated with outcome in critically ill patients requiring RRT. Finally, it must also be pointed out that our study population was more critically ill than those described by previous studies on AKI [5, 25, 43], as evidenced by high average APACHE II scores, both at initiation of RRT and ICU admission (33 and 29 points). This fact might also explain the unusually low ratios of observed to predicted mortality. In future studies, we will compare the predictive ability of circulating Ang-2 with additional AKI scores (e.g., Cleveland clinic score) and urinary biomarkers of AKI (e.g., NGAL) in a longitudinal fashion.
In summary, the results from this study indicate that circulating Ang-2 is as a strong and independent predictor of mortality in ICU patients with dialysis-dependent AKI. Given the limited value of severity-of-illness scoring, circulating Ang-2 may serve as a valuable biomarker in critical care nephrology.
References
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C (2005) Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813–818
Silvester W, Bellomo R, Cole L (2001) Epidemiology, management, and outcome of severe acute renal failure of critical illness in Australia. Crit Care Med 29:1910–1915
Douma CE, Redekop WK, van der Meulen JH, van Olden RW, Haeck J, Struijk DG, Krediet RT (1997) Predicting mortality in intensive care patients with acute renal failure treated with dialysis. J Am Soc Nephrol 8:111–117
Chertow GM, Soroko SH, Paganini EP, Cho KC, Himmelfarb J, Ikizler TA, Mehta RL (2006) Mortality after acute renal failure: models for prognostic stratification and risk adjustment. Kidney Int 70:1120–1126
Maccariello E, Soares M, Valente C, Nogueira L, Valenca RV, Machado JE, Rocha E (2007) RIFLE classification in patients with acute kidney injury in need of renal replacement therapy. Intensive Care Med 33:597–605
Schaefer JH, Jochimsen F, Keller F, Wegscheider K, Distler A (1991) Outcome prediction of acute renal failure in medical intensive care. Intensive Care Med 17:19–24
Fiedler U, Augustin HG (2006) Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol 27:552–558
van Meurs M, Kumpers P, Ligtenberg JJ, Meertens JH, Molema G, Zijlstra JG (2009) Bench-to-bedside review: angiopoietin signalling in critical illness—a future target? Crit Care 13:207
Wong AL, Haroon ZA, Werner S, Dewhirst MW, Greenberg CS, Peters KG (1997) Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ Res 81:567–574
Wakui S, Yokoo K, Muto T, Suzuki Y, Takahashi H, Furusato M, Hano H, Endou H, Kanai Y (2006) Localization of Ang-1, -2, Tie-2, and VEGF expression at endothelial-pericyte interdigitation in rat angiogenesis. Lab Invest 86:1172–1184
Fiedler U, Krissl T, Koidl S, Weiss C, Koblizek T, Deutsch U, Martiny-Baron G, Marme D, Augustin HG (2003) Angiopoietin-1 and angiopoietin-2 share the same binding domains in the Tie-2 receptor involving the first Ig-like loop and the epidermal growth factor-like repeats. J Biol Chem 278:1721–1727
Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD (1996) Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87:1161–1169
Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277:55–60
Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD (1996) Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87:1171–1180
Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG (2006) Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 12:235–239
Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG (2005) The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci 118:771–780
Kumpers P, van Meurs M, David S, Molema G, Bijzet J, Lukasz A, Biertz F, Haller H, Zijlstra JG (2009) Time course of angiopoietin-2 release during experimental human endotoxemia and sepsis. Crit Care 13:R64
Orfanos SE, Kotanidou A, Glynos C, Athanasiou C, Tsigkos S, Dimopoulou I, Sotiropoulou C, Zakynthinos S, Armaganidis A, Papapetropoulos A, Roussos C (2007) Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit Care Med 35:199–206
Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP (2006) Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 3:e46
Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG (2004) The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103:4150–4156
Kumpers P, Lukasz A, David S, Horn R, Hafer C, Faulhaber-Walter R, Fliser D, Haller H, Kielstein JT (2008) Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care 12:R147
Faulhaber-Walter R, Hafer C, Jahr N, Vahlbruch J, Hoy L, Haller H, Fliser D, Kielstein JT (2009) The Hannover Dialysis Outcome study: comparison of standard versus intensified extended dialysis for treatment of patients with acute kidney injury in the intensive care unit. Nephrol Dial Transplant 24:2179–2186
Kielstein JT, Kretschmer U, Ernst T, Hafer C, Bahr MJ, Haller H, Fliser D (2004) Efficacy and cardiovascular tolerability of extended dialysis in critically ill patients: a randomized controlled study. Am J Kidney Dis 43:342–349
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P (2004) Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212
Joannidis M, Metnitz B, Bauer P, Schusterschitz N, Moreno R, Druml W, Metnitz PG (2009) Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med 35:1692–1702
Vincent JL, Moreno R, Takala J, Willatts S, de Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International sepsis definitions conference. Intensive Care Med 29:530–538
Lukasz A, Hellpap J, Horn R, Kielstein JT, David S, Haller H, Kumpers P (2008) Circulating angiopoietin-1 and angiopoietin-2 in critically ill patients: development and clinical application of two new immunoassays. Crit Care 12:R94
Cox DR (1972) Regression models and life-tables. J R Stat Soc B34:187–202
van der Heijden M, Pickkers P, Nieuw Amerongen GP, van Hinsbergh VW, Bouw MP, van der Hoeven JG, Groeneveld AB (2009) Circulating angiopoietin-2 levels in the course of septic shock: relation with fluid balance, pulmonary dysfunction and mortality. Intensive Care Med 35:1567–1574
Gallagher DC, Parikh SM, Balonov K, Miller A, Gautam S, Talmor D, Sukhatme VP (2008) Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock 29:656–661
Su L, Zhai R, Sheu CC, Gallagher DC, Gong MN, Tejera P, Thompson BT, Christiani DC (2009) Genetic variants in the angiopoietin-2 gene are associated with increased risk of ARDS. Intensive Care Med 35:1024–1030
Roviezzo F, Tsigkos S, Kotanidou A, Bucci M, Brancaleone V, Cirino G, Papapetropoulos A (2005) Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther 314:738–744
Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA (2003) Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol 285:F191–F198
Kuo MC, Patschan D, Patschan S, Cohen-Gould L, Park HC, Ni J, Addabbo F, Goligorsky MS (2008) Ischemia-induced exocytosis of Weibel-Palade bodies mobilizes stem cells. J Am Soc Nephrol 19:2321–2330
Kumpers P, Hellpap J, David S, Horn R, Leitolf H, Haller H, Haubitz M (2009) Circulating angiopoietin-2 is a marker and potential mediator of endothelial cell detachment in ANCA-associated vasculitis with renal involvement. Nephrol Dial Transplant 24:1845–1850
Kumpers P, David S, Haubitz M, Hellpap J, Horn R, Brocker V, Schiffer M, Haller H, Witte T (2008) The Tie2 receptor antagonist Angiopoietin-2 facilitates vascular inflammation in Systemic Lupus Erythematosus. Ann Rheum Dis. doi:10.1136/ard.2008.094664
Ganter MT, Cohen MJ, Brohi K, Chesebro BB, Staudenmayer KL, Rahn P, Christiaans SC, Bir ND, Pittet JF (2008) Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg 247:320–326
Giuliano JS Jr, Lahni PM, Harmon K, Wong HR, Doughty LA, Carcillo JA, Zingarelli B, Sukhatme VP, Parikh SM, Wheeler DS (2007) Admission angiopoietin levels in children with septic shock. Shock 28:650–654
Giuliano JS Jr, Lahni PM, Bigham MT, Manning PB, Nelson DP, Wong HR, Wheeler DS (2008) Plasma angiopoietin-2 levels increase in children following cardiopulmonary bypass. Intensive Care Med 34:1851–1857
Siner JM, Bhandari V, Engle KM, Elias JA, Siegel MD (2009) Elevated serum angiopoietin 2 levels are associated with increased mortality in sepsis. Shock 31:348–353
Bagshaw SM, George C, Bellomo R (2008) A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant 23:1569–1574
Acknowledgments
We are indebted to our ICU colleagues at the Medical School Hannover for intensive monitoring of the patients.
Conflict of interest statement
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
P. Kümpers and C. Hafer contributed equally to the manuscript and are both considered first authors.
J. T. Kielstein and R. Faulhaber-Walter contributed equally to the manuscript and are both considered last authors.
Philipp Kümpers is the owner of www.angiopoietin.de.
This article is discussed in the editorial available at: doi:10.1007/s00134-009-1733-8.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kümpers, P., Hafer, C., David, S. et al. Angiopoietin-2 in patients requiring renal replacement therapy in the ICU: relation to acute kidney injury, multiple organ dysfunction syndrome and outcome. Intensive Care Med 36, 462–470 (2010). https://doi.org/10.1007/s00134-009-1726-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1726-7