Abstract
Objective
To describe the time course of the changes in pulmonary and vascular function, and systemic inflammation induced by injurious mechanical ventilation.
Design
Experimental study in an animal model of ventilator-induced lung injury.
Setting
Animal research laboratory.
Methods
Anesthetized male adult Sprague-Dawley rats were ventilated with VT 9 ml/kg and PEEP 5 cm H2O, or VT 35 ml/kg and zero PEEP for 1 h, and were killed. Other rats received ventilation for 1 h with high VT, to observe survival (n = 36), or to be monitored and killed at different points in time (24, 72 and 168 h; n = 7 in each group). Blood samples for measuring biochemical parameters were obtained. Post-mortem, a bronchoalveolar lavage (BAL) was performed, the aorta and pulmonary microvessels were isolated to examine ex-vivo vascular responses and pulmonary slices were examined (light microscopy).
Measurements and results
Mortality in rats ventilated with high VT was 19 of 36 (54%). Mechanical ventilation was associated with hypotension, hypoxaemia and membrane hyaline formation. AST, ALT, IL-6, MIP-2 serum and BAL fluid concentrations, as well as VEGF BAL fluid concentration, were increased in rats ventilated with high VT. Lung injury score was elevated. Aortic vascular responses to acetylcholine and norepinephrine, and microvascular responses to acetylcholine, were impaired. These changes resolved by 24–72 h.
Conclusions
Injurious ventilation is associated with respiratory and vascular dysfunction, accompanied by pulmonary and systemic inflammation. The survival rate was about 50%. In survivors, most induced changes completely normalized by 24–72 h after the insult.
Similar content being viewed by others
Introduction
Mechanical ventilation using high tidal volumes (VT) causes pulmonary inflammation and high permeability pulmonary oedema (ventilator induced lung injury, VILI) [1, 2]. Recent studies have shown that pulmonary dysfunction in VILI is accompanied by systemic inflammation and dysfunction of other organs [3–14]. High tidal volume ventilation has been shown to cause systemic inflammation [5], intestinal and renal apoptosis [7], renal dysfunction [8], vascular dysfunction [9–11] and intestinal hyperpermeability [12].
We hypothesized that severe VILI leads to changes in the structure and function of the lung that, despite discontinuation of the insult, result in overwhelming mortality. In those animals that survive otherwise fatal biophysical injury, the process of repair ensues and over time changes associated with VILI are reversed.
Advancement in the knowledge of this field is clinically pertinent and of translational relevance as understanding the process of recovery (repair, survival or resistance to injury) becomes important in trying to identify novel therapies for VILI. Therapeutic alternatives that accelerate repair or promote resistance (similar to that seen in survivors) can become an option for treatment in the future.
Material and methods
Animals and instrumentation
Animals were treated following the Principles of Laboratory Animal Care (UE 609/86 CEE, Real Decreto 223/88 BOE-18/03). Male Sprague-Dawley rats (Universidad Autónoma de Madrid), weighing (mean ± SD) 340 ± 15 g, were anesthetized with i.p. boluses of diazepam (15 mg/kg) and ketamine (75 mg/kg) as needed. A surgical tracheotomy was performed and a 14-G cannula inserted and connected to a mechanical ventilator (Babylog 8000 Plus, Dräger, Lübeck, Germany). An arterial catheter (Pd 23, Gould, Cleveland, OH, USA) was inserted into the left carotid artery, and another catheter (G18, Vygon, Ecouen, France) was introduced into the oesophagus. A small laparotomy was performed and the abdominal aorta was carefully dissected to place a Doppler blood flow probe around the aorta just above the renal artery.
Protocol
Rats were randomly assigned to two ventilation groups: the control group, ventilated with the protective (low VT, PEEP = 5 cm H2O) strategy (n = 7); and the overventilated group, ventilated with the injurious (high VT, PEEP = 0 cm H2O) strategy (n = 7). In both groups respiratory rate was 67 bpm (inspiratory time 0.3 s, expiratory time 0.6 s), and FiO2 0.45. Dead space ventilation was increased in animals ventilated with high VT to attain comparable values of PaCO2 in the two groups.
After 1 h of ventilation, VT was set at 9 ml/kg in all rats, and measurements (see below) were performed under these ventilatory conditions. Thereafter, rats in these two groups were killed.
Other rats received ventilation for 1 h with high VT, had their tracheotomy closed and sutured, and were sent back to their cages breathing room air. Long-term survival was observed every 6 h in 36 rats.
Additional rats were ventilated for 1 h to be monitored and killed at 24, 72 and 168 h, until a sample size of n = 7 in each group was completed. At the specified points in time, rats were anesthetized, had their tracheostomy re-opened, were intubated, ventilated with VT = 9 ml/kg and PEEP = 5 mmHg, and instrumented as indicated above. Measurements and blood sampling were performed after a 10-min stabilization period and then rats were killed by exsanguination. No fluid resuscitation was administered during the experiments.
Post-mortem, a bronchoalveolar lavage (BAL) was performed, by instilling 5 ml of saline. The aorta was removed and pulmonary microvessels were carefully dissected from the right lower lobe.
Measurements
Haemodynamic and respiratory measurements
Mean arterial blood pressure (MAP, Hewlett Packard, model 66s, Midvale, UT, USA), aortic blood flow (QAo, Transonics Systems, Ithaca, NY, USA), and oesophageal pressure (PES) were measured. Positive end-expiratory pressure (PEEP), peak inspiratory pressure (PIP), mean airway pressure (PAW), VT and respiratory system compliance were monitored using ventilator transducers. Systemic vascular resistance was estimated by the quotient MAP/QAo. All measurements were made at VT 9 ml/kg.
Biochemical measurements
Arterial blood gases (Gem Premier 3000, IL Instrumentation Laboratory, Barcelona, Spain), lactate concentration, aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH; Integra 700, Roche Diagnostics, Basel, Switzerland) and vascular endothelial growth factor (VEGF), macrophage inhibitory protein (MIP)-2 and interleukin (IL)-6 serum concentrations (rat-specific immunoassay kits, R&D Systems, Minneapolis, MN, USA) were determined. This assay measures biologically active murine VEGF120 and VEGF164, and we cannot exclude the possibility that other biologically active forms of VEGF were altered in the experiment. The BAL fluid was used for the determinaton of red blood cell (RBC) and white blood cell (WBC) count, and protein, AST, ALT, LDH, IL-6, VEGF and MIP-2 concentrations.
Histological analysis
Lung tissue from all study groups and from non-survivors after the injurious mechanical ventilation strategy were analysed. The right lung was used to measure the wet/dry weight ratio (W/D) [15]. The left lung was removed and expanded with intratracheal instillation of 10% formaldehyde with a pressure of 20 cm H2O. Findings under light microscopy were assessed by two independent pathologists, blinded as per the study group. A modified lung injury score [16] was used. Ten random high-power fields for each section were examined under light microscopy (hematoxylin and eosin, 40×), and a score of 0, 1, 2 or 3 was assigned if none, one, two or more than two membranes were found, respectively. The mean of all values of the examined fields was assigned as a measure of the degree of lung injury.
Isometric tension recording of aortic segments and pulmonary microvessels
For isometric tension recording vascular rings (six aortic rings and four pulmonary microvascular rings per rat) were mounted in organ baths (KHS at 37°C, bubbled with 95% O2 and 5% CO2, pH 7.40). Microvessels were studied in a Mulvany myograph. The technician analyzing vascular responses was blind as per the study group. Transducer outputs were amplified and recorded in a polygraph (model FTO3, Grass Instruments, Quincy, Mass., USA). Vessels were stretched to their optimal resting tension. The functional integrity of each vascular ring was checked by exposing the tissue to potassium 75 mM. Responses to norepinephrine (1 nM to 10 μM) and acetylcholine (10 nM to 10 μM) were studied in resting and norepinephrine-precontracted vessels, respectively.
Statistical methods
Means between the different groups were compared by one-way ANOVA and post-hoc least significant difference (LSD) test (SPSS version 11.0). Univariate an multivariate backward linear regression analysis was performed to determine the correlation between several independent variables and a given dependent variable. Values are means ± SEM. Significance was considered at a p-value of less than 0.05.
Results
Survival
For the survival study, 36 rats underwent high VT ventilation. Mortality is this group was 19 (54%), and the remaining 17 survived 168 h All non-survivors died during the first 6 h Other rats (n = 53) underwent high VT ventilation, to form groups that were to be killed at different points in time. Seven rats were used for the group killed at 1 h; of the remaining 46, 21 survived to form groups killed at 24, 72 and 168 h (n = 7 at each point in time).
Haemodynamic parameters
Mean arterial pressure was significantly lower in rats ventilated with high VT than in controls at 1, 24 and 72 h after the insult. Aortic blood flow was lower than in controls only at 1 h (Table 1). Estimated vascular resistance was high at 1 h.
Respiratory system mechanics
Rats ventilated with high VT showed at 1 and 24 h after starting mechanical ventilation a significant increase in PIP. Mean PAW was higher and respiratory system compliance was lower than in the control group only at 1 h. The wet/dry weight ratio was increased at 1 h and remained higher than baseline throughout the observation period (Table 1).
Gas exchange and selected biochemical measurements
Rats ventilated with high VT showed 1 h after starting mechanical ventilation impaired gas exchange, whereas oxygenation was normal after extubation. There was arterial blood acidosis at 1 h, and bicarbonate was lower than in controls from 1 to 168 h. Serum creatinine concentration increased slightly but significantly over time (Table 1).
Serum biochemical and inflammatory markers
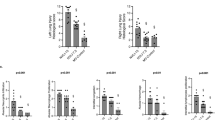
Rats ventilated with high VT showed, 1 h after starting mechanical ventilation, higher serum levels of AST, ALT, LDH, IL-6 and MIP-2 as compared with the control group, and normalized thereafter. No differences in VEGF levels were found in the different groups (Fig. 1; Table 2).
Serum and BAL fluid concentrations of interleukin-6 (IL-6), macrophage inhibitory protein-2 (MIP-2) and vascular endothelial growth factor (VEGF). Study groups as indicated in Table 1. All values are pg/ml. Data are mean ± SE. *p < 0.05 vs. control group
BAL fluid biochemical and inflammatory markers
Rats ventilated with high VT showed, 1 h after starting mechanical ventilation, higher BAL fluid concentration of proteins, AST, ALT, LDH, IL-6, MIP-2 and a greater number of RBCs (Tables 1, 2) as compared with the control group. All these measurements were not different from controls at 24 h, except for IL-6 and RBCs, which remained significantly high at 72 h. The VEGF BAL fluid only increased at 24 h, and at 72 h levels were normal (Fig. 1; Table 1, 2).
ALT (at 1 h), IL-6 (at 72 h) and VEGF (at 24 h) BAL fluid/serum concentration ratios were higher than baseline.
Vascular function
Aortic vessels
There was a significant impairment in the vascular responses to both norepinephrine (p < 0.01) and acetylcholine (p < 0.001) at 1, 24 and 72 h, as compared with the control group. Responses at 168 h were normal (Fig. 2).
Vascular reactivity to norepinephrine and acetylcholine. a Norepinephrine-induced contractions of resting aortic rings. b Acetylcholine-induced relaxations of norepinephrine pre-contracted aortic rings. c Acetylcholine-induced relaxations of norepinephrine pre-contracted pulmonary microvessels. Study groups as indicated in Table 1. Data are mean ± SE. *p < 0.05 vs. control group
Pulmonary microvessels
Vascular responses to acetylcholine were normal at 1 h; however, there was a significant impairment (p < 0.05) at 24 h, as compared with the control group. Responses normalized at 72 h (Fig. 2).
Histology
At light microscopy, capillary congestion, interstitial oedema, type-I pneumocyte necrosis and hyaline membrane formation covering the denuded epithelial surface were present in all lungs at 1 h, and in 2 of 14 from the group killed at 24 h (Fig. 3). No significant changes were observed at 72 and 168 h.
The lung injury score was significantly higher than in controls at 1 h, but the difference did not reach statistical significance at 24 h (Table 1). Non-survivors showed a comparable degree of morphological injury as survivors at 1 h (21 ± 3 vs. 22 ± 3, respectively, p = 0.71).
Relationship between cytokine concentrations and haemodynamic, pulmonary and renal parameters
The MAP correlated significantly with IL-6 (r 2 = 0.43, p < 0.001) and MIP-2 BAL fluid concentration (r 2 = 0.25, p = 0.005), but not with IL-6, MIP-2 or VEGF serum concentration or VEGF BAL fluid concentration. In multivariate analysis, including in the model all cytokines measured, only IL-6 BAL fluid concentration was significantly associated with MAP (r 2 = 0.48, p = 0.001).
Lung injury score correlated with IL-6 serum concentration (r 2 = 0.33, p = 0.001) as well as with MIP-2 serum (r 2 = 0.40, p < 0.001) and BAL fluid concentration (r 2 = 0.30, p = 0.002), but not with IL-6 BAL fluid concentration or VEGF serum or BAL fluid concentration. In multivariate analysis, including in the model all measured cytokines, only IL-6 serum concentration (r 2 = 0.61, p < 0.001) was associated with the lung injury score.
Serum creatinine concentration correlated with MAP (r 2 = 0.30, p = 0.001) and MIP-2 serum concentration (r 2 = 0.19, p = 0.02). In a multivariate model MAP, and including all other measured cytokines (r 2 = 0.39, p = 0.001), MAP (p = 0.003), but not MIP-2 serum concentration (p = 0.059), was associated with serum creatinine concentration.
Discussion
The main finding of this study is that the pulmonary and cardiovascular injuries, as well as the accompanying release of local and systemic inflammatory mediators induced by injurious mechanical ventilation, were reversible 24–72 h after the insult was discontinued.
The ventilatory strategy used was associated with membrane hyaline formation in the lungs, worsened gas exchange, hypotension, elevation of serum AST, ALT and LDH, renal dysfunction, vascular dysfunction in pulmonary and extra pulmonary vessels, and increased systemic (IL-6, MIP-2 in the serum) and lung (IL-6, MIP-2, VEGF in the BAL fluid) inflammatory mediators. Although it is known that injurious mechanical ventilation induces pulmonary and systemic inflammation [3–12], the time course and reversibility of these abnormalities have not been previously analyzed; thus, as described below, this model can be portrayed as a model of pulmonary epithelial cell injury, loss of compartimentalization of the inflammatory response and VILI-induced shock and recovery.
Pulmonary function and morphological changes
Injurious mechanical ventilation was associated with impairment in gas exchange, a decrease in respiratory system compliance and membrane hyaline formation. These findings are in keeping with previous studies showing pulmonary inflammation after high VT ventilation [2–8, 10]. In the context of high VT-induced mechanical disruption of the pulmonary parenchyma followed by inflammation, there was an increase in BAL fluid RBC count and protein concentration at 1 h after starting mechanical ventilation. We cannot explain the elevation of peak PAW at 24 h, after decreasing VT, coinciding with a normalization of mean PAW. This could be due to increased airway resistance as a result of oedema (persistent increased lung wet/dry weight ratio).
Morphological changes as seen at light microscopy completely disappeared at 72 h, and no abnormalities or signs of tissue fibrosis could be observed at this point in time or thereafter.
Changes in inflammatory mediators and markers of cell injury
The increase in AST, ALT, LDH, IL-6 and MIP-2 serum concentrations indicate cell damage and systemic inflammation induced by injurious mechanical ventilation. Changes consistent with cell damage and pulmonary inflammation were also observed in the lung.
The high BAL fluid/serum concentration ratios of IL-6 and VEGF suggest pulmonary production of these cytokines, with loss of compartimentalization of the inflammatory response. It is also possible that these mediators are leaking into the lung from the circulation and simply not being cleared.
Our findings of systemic and pulmonary cytokine release, accompanied by pulmonary oedema and membrane hyaline formation, are in line with previous knowledge about the biological changes related to VILI [2, 17–20]. The correlation we found between the lung injury score and IL-6 serum concentration favours the hypothesis of the systemic pro-inflammatory role of the injured lung.
In our study, increased VEGF BAL levels in rats undergoing injurious mechanical ventilation might increase vascular permeability, coinciding with the increase in IL-6 BAL fluid levels, or might reflect an ongoing vascular repair process [21–26]; however, as we did not find a correlation between VEGF concentrations (either in the serum or in the BAL fluid) and lung injury score, IL-6 or PaO2 (data not given), we cannot favour any of the above-mentioned hypotheses.
Renal function
Serum creatinine concentration was very slightly, but significantly, increased up to 72 h in animals receiving high VT ventilation. The correlation of this change with MAP points toward a possible pre-renal origin of this slight change in serum creatinine, although, given the almost significant correlation with MIP-2 serum concentration, we cannot rule out systemic inflammation-induced renal dysfunction, as has been previously reported [8].
Vascular function
We found in rats undergoing injurious ventilation hypotension and, ex vivo, decreased contractile response of aortic rings, and impaired relaxation of both aortic and pulmonary microvascular rings.
The early (at 1 h) hypotension in our study could be explained by haemodynamic changes related to large swings in intrathoracic pressure; however, the progressive nature of the blood pressure decline observed during 1 h of injurious mechanical ventilation, the absence of significant changes in oesophageal pressure, and the persistence of the hypotension at 24 and 72 h (measured under conditions of low tidal volume ventilation) argue against a primarily haemodynamic effect explaining the hypotension.
The hypotension and the ex-vivo vascular dysfunction could be explained by the inflammatory response induced by the injurious mechanical ventilation. A mechanical effect on the vessels studied, related to cyclic changes in intrathoracic mechanical conditions, could also explain the abnormal ex-vivo vascular function. We cannot prove a cytokine-mediated loss of vascular tone as the cause of hypotension, as the decrease in SVR at 24 and 72 h as compared with baseline did not reach statistical significance; however, we think that hypotension was due to vasodilation rather than to decreased systemic blood flow, since QAo at these points in time was similar to baseline. Hypotension associated with injurious mechanical ventilation has been previously described [10, 27]. The mechanisms of the reported vascular dysfunction were not specifically addressed in the present study. The correlation between MAP and IL-6 BAL fluid concentration suggests, but does not prove, the role of the injured lung in vascular dysfunction in this model.
Limitations of the study
Although our design (temporary mechanical ventilation insult followed by extubation and observation for a period of time) has no immediate clinical correlate, it does allow for the observation of the time course of several physiological, biochemical and histological changes.
The observation of changes over time in (surviving) rats is inevitably associated with a survival bias.
We chose as controls animals ventilated with low VT for 1 h, as the assumption that these non-injured animals would not show changes over time was reasonable. Admittedly, a more appropriate approach would have been to re-intubate these animals (low VT group) at different points in time for comparison with the high VT group.
Our study describes the time pattern of biochemical and some organ function changes after a pulmonary insult, and the mechanism of death has not been addressed. The lack of morphological analysis of non-pulmonary organs in the present study limits our knowledge of the extension of organ damage and the cause of death in VILI.
As no specific infection prevention measures were undertaken, the vascular abnormalities described could in theory be explained by infection.
Our study design did not allow us to determine the cause of death among non-survivors in this model of VILI. The comparable degree of lung morphological injury in non-survivors at the time of death (occurring from 1 to 6 h) and in survivors at 1 h does not support respiratory failure as the cause of death, although this is still possible. The remaining injury in survivors could represent shock, in the context of cytokine release, or perhaps bacterial translocation from the gut or the lung, or ischemia-reperfusion injury.
Conclusion
In conclusion, we report respiratory and vascular dysfunction induced by injurious ventilation, accompanied by pulmonary and systemic inflammation. In survivors, most of the observed changes are transient and normalize by 24–72 h after the insult, whereas others (pulmonary oedema, and acidosis) persisted for the entire observation period. More studies are needed to investigate interventions that modulate the response to injurious ventilation, as well as the mechanism of death.
References
Dreyfuss D, Basset G, Soler P, Saumon G (1985) Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am Rev Respir Dis 132:880–884
Dreyfuss D, Saumon G (1998) Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157:294–323
von Bethmann AN, Brasch F, Müller K, Vogt K, Volk HD, Muller KM, Wendel A, Uhlig S (1996) Prolonged hyperventilation is required for release of tumor necrosis factor but not IL-6. Appl Cardiop Pathophysiol 16:171–177
Held HD, Boettcher S, Hamann L, Uhlig S (2001) Ventilation-induced chemokine and cytokine release is associated with activation of nuclear factor-kB and is blocked by steroids. Am J Respir Crit Care Med 163:771–716
Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS (1997) Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 99:944–952
Chiumello D, Pristine G, Slutsky AS (1999) Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory syndrome. Am J Respir Crit Care Med 160:109–116
Imai Y, Kajikawa I, Frevert C, de Perrot M, Fischer F, Parodo J, Edwards V, Cutz E, Zhang H, Ranieri M, Liu M, Keshavjee S, Marshall JC, Martin TR, Slutsky AS (2003) Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. J Am Med Assoc 289:1204–2112
Choi WI, Quinn DA, Park KM, Moufarrej RK, Jafari B, Syrkina O, Bonventre JV, Hales CA (2003) Systemic microvascular leak in an in vivo rat model of ventilator-induced lung injury. Am J Resp Crit Care Med 167:1627–1632
Nin N, Valero JA, Lorente JA, de Paula M, Fdez-Segoviano P, Sánchez-Ferrer A, Esteban A (2005) Large tidal volume ventilation induces vascular dysfunction in rats. J Trauma 59:711–716
Nin N, Penuelas O, de Paula M, Lorente JA, Fernández-Segoviano P, Esteban A (2006) Ventilation-induced lung injury in rats is associated with organ injury and systemic inflammation that is attenuated by dexamethasone. Crit Care Med 34:1093–1098
Mandava S, Kolobow T, Vitale G, Feti G, Aprigliano M, Jones M, Muller E (2003) Lethal systemic capillary leak syndrome associated with severe ventilator-induced lung injury: an experimental study. Crit Care Med 31:885–892
Guery BP, Welsh DA, Viget NB, Robriquet L, Fialdes P, Mason CM, Beaucaire G, Bagby GJ, Neviere R (2003) Ventilation-induced lung injury is associated with an increase in gut permeability. Shock 19:559–563
Ranieri VM, Suter PM, Tortorella C, de Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky A (1999) Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome J Am Med Assoc 282:54–61
Ranieri VM, Giunta F, Suter PM, Slutsky AS (2000) Mechanical ventilation as a mediator of multisystem organ failure in acute respiratory distress syndrome J Am Med Assoc 284:43–44
Selinger SL, Bland RD, Demling RH, Staub NC (1970) Distribution volumes of [131I]albumin, [14C]sucrose, and 36Cl in sheep lung. J Appl Physiol 39:773–779
Muscedere JG, Mullen JB, Gan K, Slutsky AS (1994) Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 149:1327–1334
Mascarenhas MM, Day RM, Ochoa CD, Choi WI, Yu L, Ouyang B, Garg HG, Hales CA, Quinn DA (2004) Low molecular weight hyaluronan from stretched lung enhances interleukin-8 expression. Am J Respir Cel Mol Biol 30:51–60
Quinn DA, Tager AM, Joseph P, Bonventre J, Force T, Hales CA (1999) Stretch-induced mitogen-activated protein kinase activation and IL-8 production in type-2 alveolar cells. Chest 116:89S–90S
Liu M, Transwell K, Post M (1999) Mechanical force-induced signal transduction in lung cells. Am J Physiol 277:L667–L683
Pan J, Fukuda K, Saito M, Matsuzaki J, Kodama H, Sano M, Takahashi T, Kato T, Ogawa S (1999) Mechanical stretch activates the JAK/STAT pathway in rat cardiomyocytes. Circulation Res 84:1127–1136
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676
Mittermayer F, Pleiner J, Schaller G, Weltermann A, Kapiotis S, Jilma B, Woltz M (2003) Marked increase in vascular endothelial growth factor concentrations during Escherichia coli endotoxin-induced acute inflammation in humans. Eur J Clin Invest 33:758–761
Thickett DR, Armstrong L, Christie SJ, Millar AB (2001) Vascular endothelial growth factor may contribute to increased vascular permeability in acute respiratory distress syndrome. Am J Respir Crit Care Med 164:1601–1605
Eppler SM, Combs DL, Henry TD, Lopez JJ, Ellis SG, Yi JH, Annex BH, McCluskey ER, Zioncheck TF (2002) A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin Pharmacol Ther 5:107–110
Mura M, dos Santos CC, Stewart D, Liu M (2004) Vascular endothelial growth factor and related molecules in acute lung injury. J Appl Physiol 97:1605–1617
Ohwada A, Yoshioka Y, Iwabuchi K, Nagaoka I, Dambara T, Fukuchi Y (2003) VEGF regulates the proliferation of acid-exposed alveolar lining epithelial cells. Thorax 58:328–332
Borelli M, Kolobow T, Spatola R, Prato P, Tsuno K (1988) Severe acute respiratory failure managed with continuous positive airway pressure and partial extracorporeal carbon dioxide removal by an artificial membrane lung. Am Rev Respir Dis 138:1480–1487
Acknowledgements
This study was funded by grant FIS 03/1340, RED RESPIRA (RTIC C03/11), FIS 06/1664.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: http://dx.doi.org/10.1007/s00134-007-0961-z.
Rights and permissions
About this article
Cite this article
Nin, N., Lorente, J.A., de Paula, M. et al. Rats surviving injurious mechanical ventilation show reversible pulmonary, vascular and inflammatory changes. Intensive Care Med 34, 948–956 (2008). https://doi.org/10.1007/s00134-007-0959-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0959-6