Abstract
Objective
The objective was to prospectively evaluate cardiac morphological and functional changes using transesophageal echocardiography (TEE) during early septic shock.
Design
Prospective, observational study.
Setting
Medical-surgical intensive care unit of a teaching hospital.
Patients and participants
Ventilated patients with septic shock, sinus rhythm and no cardiac disease underwent TEE within 12 h of admission (Day 0), after stabilization of hemodynamics by fluid loading (median volume: 4.9 l [lower and upper quartiles: 3.7–9.6 l]) and vasopressor therapy, and after vasopressors were stopped (Day n).
Measurements and results
Thirty-five patients were studied (median age: 60 years [range 44–68]; SAPS II: 53 [46–62]; SOFA score: 9 [8–11]) and 9 of them (26%) died while on vasopressors. None of the patients exhibited TEE findings of cardiac preload dependence. Between Day 0 and Day n (7 days [range 6–9]), mean left ventricular (LV) ejection fraction (EF) increased (47 ± 20 vs. 57 ± 14%: p < 0.05), whereas mean LV end-diastolic volume decreased (97 ± 25 vs. 75 ± 20 ml: p < 0.0001). Out of 16 patients (46%) with LV systolic dysfunction on Day 0, 12 had normal LVEF on Day n and 4 patients fully recovered by Day 28. Only 4 women had LV dilatation (range, LV end-diastolic volume: 110–148 ml) on Day 0, but none on Day n. Doppler tissue imaging identified an LV diastolic dysfunction in 7 patients (20%) on Day 0 (3 with normal LVEF), which resolved on Day n.
Conclusions
This study confirms that LV systolic and diastolic dysfunctions are frequent, but LV dilatation is uncommon in fluid-loaded septic patients on vasopressors. All abnormalities regressed in survivors, regardless of their severity.
Descriptors
Shock: clinical studies (38), Cardiovascular monitoring (34).
Similar content being viewed by others
Introduction
Circulatory failure associated with septic shock is characterized by marked hypovolemia (absolute, secondary to increased capillary permeability and relative, due to vasoplegia) and an intrinsic ventricular dysfunction partially masked by decreased vascular resistance [1–3]. Echocardiography Doppler, especially using the transesophageal route (TEE), is increasingly used in intensive care unit (ICU) settings for the assessment of patients presenting with circulatory failure [4]. By providing morphological as well as functional real-time information, TEE is ideally suited to promptly identifying the mechanisms of sepsis-induced hypotension in ventilated ICU patients [5].
Transient left ventricular (LV) systolic dysfunction has long been identified during the acute phase of septic shock using right heart catheterization [6] or echocardiography [7]. The presence of reversible LV structural changes, suggesting preload adaptation, have also been described [3, 6], but remains controversial [8]. In addition, LV diastolic dysfunction has been reported during septic shock [9–11], but these descriptions relied on the use of conventional pulse wave Doppler parameters, which are sensitive to rapid changes in loading conditions, such as fluid therapy and vasopressor administration [12]. Finally, right ventricular (RV) dysfunction has been observed in up to 30% in one series of septic shock patients assessed using TEE [5], but has otherwise been scarcely reported.
The present study was aimed at prospectively evaluating both cardiac function and structure using TEE in ventilated patients with septic shock. Particular attention was directed toward the identification of LV diastolic dysfunction using preload-independent Doppler parameters and the assessment of potential ventricular structural changes (i. e., dilatation) during the early phase of septic shock.
Materials and methods
Patients
For 12 months, adult patients presenting to our medical-surgical ICU with septic shock were eligible to participate in this descriptive study if they fulfilled all the following criteria:
-
1.
Mechanical ventilation
-
2.
Evidence of a source of infection for < 6 days
-
3.
At least 2 of the 4 SIRS criteria, as previously defined [13]
-
4.
Two or more signs of tissue hypoperfusion of organ dysfunction out of the following: PaO2/FIO2 < 300, urinary output < 0.5 ml/kg/h for at least 2 h, arterial lactate ≥ 3 mmol/l, platelet count < 100,000/mm3 per cubic millimeter [14]
-
5.
For less than 24 h, sustained hypotension (systolic blood pressure < 90 mmHg) lasting at least 30 min that required ≥ 1 l of blood volume expansion and the administration of vasopressor therapy (norepinephrine or epinephrine)
-
6.
Stable hemodynamics after fluid loading and initiation of vasopressor therapy
Patients were excluded in the presence of at least one of the following criteria:
-
1.
Other cause of shock
-
2.
Previous cardiac disease
-
3.
Absence of sinus rhythm
-
4.
Contra-indication for performing a TEE
-
5.
Polynuclear neutrophil count < 500/mm3
-
6.
Moribund status or decision to withhold aggressive therapy
Hypovolemia was considered corrected in the presence of a respiratory variation of invasive pulse arterial pressure < 13% [15]. Associated treatment (e. g., corticosteroids, recombinant activated protein C) was left to the discretion of the attending intensivists [16]. We have long used TEE for the routine hemodynamic assessment of critically ill patients sustaining circulatory failure in our medical-surgical ICU [17]. Since we routinely monitor septic shock patients using TEE, iterative examinations are performed including at the time of vasopressor weaning. As such, the protocol was approved by the Ethics Committee of the Société de Réanimation de Langue Française that waived the need for signed informed consent.
TEE studies
In each patient, TEE was performed by experienced intensivists with a level III competence in echocardiography [18] within 12 h of admission (Day 0) in hemodynamically stable patients, and when vasopressors were stopped (Day n). In the presence of persistent abnormalities on Day n, transthoracic echocardiography (spontaneously breathing patients) was performed on Day 28 in survivors. TEE was performed as previously described [17], using a Sonos 5500 upper-end platform (Philips, France) connected to a multiplane 5-MHz TEE probe. Patients received midazolam (0.15 mg/kg) and pancuronium bromide (0.1 mg/kg) prior to esophageal probe insertion. Therapeutic changes based on real-time TEE results interpretation were recorded while digital loops and still-frames were stored on optical disks for off-line measurement and further analysis. In each patient, the following TEE parameters were measured off-line (mean of three end-expiratory measurements): LV end-diastolic and LV end-systolic volumes using the modified Simpson's rule and four-chamber area-length methods, LV stroke volume using pulse wave Doppler at the level of the LV outflow tract [19], LV and RV end-diastolic areas, and early diastolic Doppler tissue imaging (DTI) velocities (Ea) of the lateral aspect of the mitral ring in the transesophageal long axis view [12]. The LV ejection fraction (EF) was conventionally calculated and the RV/LV end-diastolic area ratio was computed. In addition, the superior vena cava collapsibility index and respiratory variation of aortic Doppler velocities were determined, as previously described [20, 21]. LV systolic dysfunction was classified as mild (40% < LVEF < 50%), moderate (20% < LVEF < 40%), or severe (LVEF < 20%). LV diastolic dysfunction was defined by a decreased Ea < 8.5 cm/s [22] and LV filling pressures were deemed elevated when E/Ea > 7.0 [23]. LV dilatation corresponded to a LV end-diastolic volume of > 170 ml for men and > 101 ml for women using the modified Simpson's rule method, and > 193 ml for men and > 136 ml for women using the four-chamber area-length method [24]. RV was considered dilated when the RV/LV end-diastolic area ratio exceeded 0.6 [25]. We have previously shown that our inter- and intra-observer variability in the measurement of echocardiographic parameters is globally < 11% [12, 26].
Statistics
All values are expressed as medians with lower and upper quartiles. On Day 0, clinical and TEE variables were compared between patients who subsequently died in the ICU and survivors using the Mann–Whitney test or Fisher'sexact test, as appropriate. In each patient who has been discharged from the ICU, the parameters studied were compared at Day 0 and at Day n using the Wilcoxon matched-pairs signed-rank test. A p value < 0.05 was considered statistically significant.
Results
During the study period, 132 of the 1,026 patients (13%) admitted to our ICU with suspected sepsis were screened for eligibility. Of 42 eligible patients with septic shock, 7 were excluded because of arrhythmia (n = 4), known cardiopathy (n = 2), or Boerhaave syndrome (n = 1). Finally, 35 patients were studied (19 men; median age: 60 years [lower and upper quartiles: 44–68]; Simplified Acute Physiologic Score [SAPS] II: 53 [46–62]; Sepsis-related Organ Failure Assessment [SOFA]: 9 [8–11]). Median duration of sepsis before TEE assessment of hemodynamics on Day 0 was 48 h (36–96 h). At the time of initial TEE examination, patients had received a median fluid volume of 4.9 l (3.7–9.6 l). The vasoactive drugs administered were norepinephrine (n = 32), epinephrine (n = 12), or both (n = 9), but none of the patients received dobutamine. Nine patients (26%) died while on vasopressors (no TEE on Day n), whereas vasoactive drugs were stopped after a median of 7 days (range 6–9 days) in the remaining 26 patients who were all discharged from the ICU. Twenty-five patients presented with a community-acquired infection whereas the 10 remaining patients had a nosocomial infection. Sites of infection were: intra-abdominal (37%), pneumonia (29%), urinary tract (11%), skin (9%), and miscellaneous (14%). The source of sepsis was documented in 25 patients (71%) and 14 patients (40%) had bacteremia. Twelve patients died during their hospital stay (34%).
Patients had frequent comorbidities, including chronic alcoholism (n = 12), cancer or hemopathy (n = 9), diabetes (n = 5), chronic renal failure (n = 3), and hypertension (n = 13). Five patients were receiving long-term treatment by beta-blockers and 7 patients by angiotensin conversion enzyme inhibitors. At the time of inclusion, patients who subsequently died during their ICU stay required higher doses of vasopressor, had greater blood lactate levels and exhibited more organ dysfunctions, as reflected by a higher SOFA score, compared with survivors (Table 1).
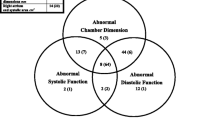
Transesophageal echocardiography findings recorded on Day 0 and Day n are summarized in Table 2. None of the patients exhibited TEE findings of cardiac preload dependence at the time of the examinations, since both the collapsibility index of the superior vena cava and the respiratory variations of aortic Doppler velocities were consistently within the lower range (Table 2). On Day 0, none of the TEE parameters allowed us to distinguish patients who would die during their ICU stay from survivors. LVEF was decreased in 16 patients (46%), reflecting a LV systolic dysfunction classified as mild in 3 patients (LVEF range: 41–49%), moderate in 7 patients (LVEF range: 24–38%), and severe in the remaining 6 patients (LVEF range: 7–18%). From Day 0 to Day n, LVEF tended to increase while LV stroke volume reached significantly greater values (Table 2). Out of the 16 patients with a depressed LV systolic function on Day 0, 12 patients had normal LV systolic performance on Day n (LVEF range: 54–82%), and the remaining 4 patients fully recovered by Day 28 (LVEF range: 57–62%). Median LV end-diastolic volume significantly decreased between the two TEE examinations (Table 2). Only 4 women had a true LV dilatation using the modified Simpson's rule (LV end-diastolic volume range: 110–148 ml) and 1 woman using the four-chamber area-length method (LV end-diastolic volume: 152 ml) on Day 0, but none on Day n (Fig. 1). All of them were alive on Day 28. Median values of Ea were similar between Day 0 and Day n (Table 2). On Day 0, DTI identified an LV diastolic dysfunction in 7 patients (20%). In this subset of patients, median Ea maximal velocity was significantly slower than that recorded in the remaining patients (7.7 cm/s [range 7.4–8.1] vs. 13.9 cm/s [range 9.1–25.7]: p < 0.0001). Isolated LV diastolic dysfunction (LVEF > 50%) was identified in 3 patients (9%) using DTI. On Day n, Ea significantly increased to reach normal values in all of those patients (14.2 cm/s [range: 9.6–32.7]: p = 0.03). Finally, 4 patients (11%) with acute respiratory distress syndrome exhibited mild RV dilatation, as reflected by anincreased RV/LV end-diastolic area ratio (range 0.7–1.0). In all cases, RV dilatation regressed with the pulmonary recovery.
Example of reversible left ventricular (LV) systolic dysfunction during the early phase of septic shock. In this patient, who received 4 l of blood volume expansion on Day 0, transesophageal echocardiography (TEE) disclosed a a normal LV end-diastolic volume and b a severe global LV systolic dysfunction reflected by a low ejection fraction and c reduced stroke volume. When vasopressors were stopped 6 days later (Day n), TEE depicted d a smaller LV cavity size and e total recovery of LV systolic performance as reflected by a normal ejection fraction and f stroke volume. LV, left ventricle; RV, right ventricle; LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; Paw, airway pressure; VTI, velocity time integral
No blood volume expansion was performed based on initial TEE study. In the presence of a moderate-to-severe LV systolic dysfunction (n = 13), inotropic support was either introduced or increased. LV diastolic dysfunction identified on the basis of reduced DTI mitral ring velocities led to fluid restriction and diuretic therapy only when associated with increased LV filling pressures in patients with acute lung injury or acute respiratory distress syndrome (n = 3). The identification of LV dilatation had a therapeutic impact only when associated with LV systolic dysfunction (n = 2). Finally, RV dilatation led to the initiation/increase in vasopressor support in conjunction with the maintenance of airway plateau pressure < 28 cm H2O (n = 4).
Discussion
This study confirms echocardiographic findings previously reported by Jardin et al. [5, 8, 27] and suggests the presence of a transient LV diastolic dysfunction using relatively preload-independent Doppler parameters. Entry criteria for participating in the present study selected the most severe patients admitted to our medical-surgical ICU for septic shock, as reflected by elevated blood lactate level and by both the number and severity of organ dysfunctions. Surprisingly, hospital mortality was fairly low in these high-risk patients, presumably due to the high proportion of intra-abdominal and urinary sepsis (48%).
Nearly half of our patients exhibited a LV systolic dysfunction during the early phase of septic shock. Importantly, TEE was initially performed after stabilization of hemodynamics obtained by substantial volume loading (median: 4.9 l [3.7–9.6]) and the administration of vasopressors. Accordingly, LV systolic dysfunction could not be ascribed to persistent hypovolemia, as reflected by the absence of TEE findings consistent with cardiac preload dependence [20, 21]. Although vasopressors may have unmasked underlying decreased myocardial contractility by increasing LV afterload [1], 34% of our patients were receiving epinephrine at the time of the initial TEE examination for clinically suspected LV systolic dysfunction. In contrast with the fairly high proportion of our patients (37%) with moderate-to-severe LV systolic dysfunction, Rivers et al. [28] previously reported a 14% incidence of LV failure in the group of patients with SCvO2 monitoring during the early course of severe sepsis and septic shock. This apparent discrepancy may be related to different population characteristics, earlier hemodynamic assessment, and mainly indirect and blind evaluation of LV performance. Our results based on direct TEE quantitative evaluation of LV systolic function corroborate previous echocardiographic studies obtained in similar clinical settings [5]. Specifically, cardiac index measured using pulsed wave Doppler at the level of the LV outflow tract [19] was < 3 l/min/m2 in 15 of our 35 patients (43%) on Day 0, similar to a proportion of 35% reported elsewhere [5]. In the majority of our surviving patients, a significant improvement in LV systolic function was observed at the time of interruption of vasopressor therapy (Day n). In all remaining patients, LV systolic performance fully recovered by Day 28, as previously reported [6, 27].
Left ventricular cavity dilatation was not observed in a large proportion of our patients, since only 4 women (11%) had an increased LV end-diastolic volume measured using the modified Simpson's rule on Day 0, and 1 woman when using the four-chamber area-length method. These results are in keeping with those reported by others in the same clinical setting [8, 11, 20, 29] and fail to corroborate the hypothesis of true LV dilatation during the early phase of septic shock in survivors [6]. Although echocardiography tends to underestimate true LV volumes compared with angiography, LV adaptation to preload is limited since its cavity cannot acutely dilate [8]. Therefore, pioneer studies that reported acute LV dilatation during the early phase of septic shock presumably overestimated actual volumes due to inherent cumulative errors relative to the combined techniques used for cavity measurements (i. e., thermodilution and radionuclide ventriculography) [3, 6, 30]. In the present study, the significant decrease in LV end-diastolic volumes between Day 0 and Day n presumably reflects the reduction in preload secondary to decreased fluid loading after the acute phase of septic shock, hence the existing yet limited LV adaptation to preload.
Left ventricular diastolic dysfunction was identified during the acute phase of septic shock in 20% of our patients. Prolonged relaxation has been documented in animal models of septic shock using invasive measurement of the time constant of LV pressure fall [31]. In septic shock patients, LV diastolic dysfunction has been previously documented using conventional pulsed wave Doppler parameters [9–11]. Unfortunately, these parameters have been shown to be markedly influenced by abrupt variations in LV preload [12], and are therefore of little value in the setting of resuscitated septic shock. In contrast, DTI velocity applied to the lateral aspect of the mitral ring appears independent of loading conditions [12]. In all our patients, DTI velocities reached normal values on Day n. Accordingly, the current data support the presence of a transient LV diastolic dysfunction, frequently associated with depressed LV systolic function, during the early phase of septic shock. The potential role of increased LV end-diastolic volume secondary to augmented preload and elevated heart rate in the development of LV diastolic dysfunction observed on Day 0 and its clinical relevance remain unclear. In the present study, the identification of LV diastolic dysfunction led to fluid restriction and diuretic therapy in 3 of the 7 patients who exhibited associated increased filling pressure with concomitant respiratory compromise.
We observed mild RV dilatation in only 4 of our septic shock patients (11%). In this setting, acute RV dysfunction may be secondary to either an intrinsic depression in RV contractility [1] or to acute cor pulmonale [5, 32]. In our patients, RV dilatation was secondary to an acute cor pulmonale, which accompanied an acute respiratory distress syndrome. Although the incidence of RV dysfunction has been reported to be as high as 32% in septic shock patients [5], we observed a lower frequency in the present study, as did others [33]. This discrepancy may be attributed to different study populations. In this specific setting, TEE is particularly valuable in guiding ventilator settings that should be protective, not only for the lungs, but also for the RV [34], as in 4 of our patients.
The present descriptive study is limited by the relatively small sample size, which precludes any definite conclusion from the comparison between deceased and surviving patients. In addition, we did not evaluate the efficacy of therapeutic changes that directly resulted from the initial TEE examination. Finally, the precise delay intotal recovery of LV systolic and diastolic function could not be precisely determined since TEE was not serially performed on a daily basis, but rather when vasoactive drugs could definitely be stopped.
Conclusions
This study confirms that LV systolic dysfunction is frequent, but initial true LV dilatation is uncommon during the acute phase of septic shock in fluid-loaded patients on vasopressors. In addition, the present study suggests that LV diastolic dysfunction assessed using a preload-independent Doppler parameter is commonly observed during the early phase of septic shock. RV dysfunction was less frequently observed and associated with acute respiratory distress syndrome. Importantly, all these changes regressed in survivors, regardless of their severity. Further studies are needed to determine whether certain echocardiographic findings could be associated with a poor prognosis in septic shock patients.
References
Grocott-Mason RM, Shah AM (1998) Cardiac dysfunction in sepsis: new theories and clinical implications. Intensive Care Med 24:286–295
Ellman H (1984) Capillary permeability in septic patients. Crit Care Med 12:629–633
Parrillo JE (1993) Pathogenetic mechanisms of septic shock. N Engl J Med 328:1471–1477
Vignon P (2005) Hemodynamic assessment of critically-ill patients using echocardiography Doppler. Curr Opin Crit Care 11:227–234
Vieillard-Baron A, Prin S, Chergui K, Dubourg O, Jardin F (2003) Hemodynamic instability in sepsis. Am J Respir Crit Care Med 168:1270–1276
Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parillo JE (1984) Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med 100:483–490
Ozier Y, Guéret P, Jardin F, Farcot JC, Bourdarias JP, Margairaz A (1984) Two-dimensional echocardiographic demonstration of acute myocardial depression in septic shock. Crit Care Med 12:596–599
Vieillard-Baron A, Schmitt JM, Beauchet A, Roch A, Prin S, Page B, Jardin F (2001) Early preload adaptation in septic shock? Anesthesiology 94:400–406
Jafri SM, Lavine S, Field BE, Bahorozian MT, Carlson MW (1990) Left ventricular diastolic function in sepsis. Crit Care Med 18:709–714
Munt B, Jue J, Gin K, Fenwick J, Tweeddale M (1998) Diastolic filling in human severe sepsis: an echocardiographic study. Crit Care Med 26:1829–1833
Poelaert J, Declerck C, Vogelaers D, Colardyn F, Visser CA (1997) Left ventricular systolic and diastolic function in septic shock. Intensive Care Med 23:553–560
Vignon P, Allot V, Lesage J, Martaillé JF, Aldigier JC, François B, Gastinne H (2007) Diagnosis of left ventricular diastolic dysfunction in the setting of acute changes in loading conditions. Crit Care 11:R43
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RMH, Sibbald WJ (1992) Definitions for sepsis organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM. Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644–1655
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G for the International Sepsis Definitions Conference (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31:1250–1656
Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky M, Teboul JL (2000) Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute respiratory failure. Am J Respir Crit Care Med 162:134–138
Dellinger RP, Carlet J, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM (2004) Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med 30:536–555
Vignon P, Mentec H, Terré S, Gastinne H, Gueret P, Lemaire F (1994) Diagnostic accuracy and therapeutic impact of transthoracic and transesophageal echocardiography in mechanically ventilated patients in the ICU. Chest 106:1829–1834
Task Force on Echocardiography in Emergency Medicine of the American Society of Echocardiography and the Echocardiography and TPEC Committees of the American College of Cardiology (1999) Echocardiography in emergency medicine: a policy statement by the American Society of Echocardiography and the American College of Cardiology. J Am Soc Echocardiogr 12:82–84
Zoghbi WA, Quinones MA (1986) Determination of cardiac output by Doppler echocardiography: a critical appraisal. Herz 11:258–268
Vieillard-Baron A, Chergui K, Rabiller A, Peyrouset O, Page B, Beauchet A, Jardin F (2004) Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med 30:1734–1739
Feissel M, Michard F, Mangin I, Ruyer O, Faller JP, Teboul JL (2001) Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest 119:867–873
Garcia MJ, Thomas JD, Klein AL (1998) New Doppler echocardiographic applications for the study of diastolic dysfunction. J Am Coll Cardiol 32:865–875
Bouhemad B, Nicolas-Robin A, Benois A, Lemaire S, Goarin JP, Rouby JJ (2003) Echocardiographic Doppler assessment of pulmonary capillary wedge pressure in surgical patients with postoperative circulatory shock and acute lung injury. Anesthesiology 98:1091–1100
Wahr DW, Wang YS, Schiller NB (1983) Left ventricular volumes determined by two-dimensional echocardiography in a normal adult population. J Am Coll Cardiol 1:863–868
Jardin F, Dubourg O, Bourdarias JP (1997) Echocardiographic patterns of acute cor pulmonale. Chest 111:209–217
Vignon P, Rambaud G, François B, Preux PM, Lang RM, Gastinne H (1998) Quantification of traumatic hemomediastinum using transesophageal echocardiography: impact on patient management. Chest 113:1475–1480
Jardin F, Brun-Ney D, Auvert B, Beauchet A, Bourdarias JP (1990) Sepsis-related cardiogenic shock. Crit Care Med 18:1055–1060
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M for the Early Goal-Directed Therapy Collaborative Group (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Tavernier B, Makhotine O, Lebuffe G, Dupont J, Scherpereel P (1998) Systolic pressure variations as a guide to fluid therapy in patients with sepsis-induced hypotension. Anesthesiology 89:1313–1321
Suffredini AF, Fromm RE, Parker MM, Brenner M, Kovacs JA, Wesley RA, Parrillo JE (1989) The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med 321:280–287
Hung J, Lew WYW (1993) Temporal sequence of endotoxin-induced systolic and diastolic dysfunction myocardial depression in rabbits. Am J Physiol 265:H810–H819
Liu D, Du B, Long Y, Zhao C, Hou Baidong (2000) Right ventricular function of patients with septic shock: clinical significance. Chin J Surg 38:488–492
Combes A, Arnoult F, Trouillet JL (2004) Tissue Doppler imaging estimation of pulmonary artery occlusion pressure in ICU patients. Intensive Care Med 30:75–81
Jardin F, Vieillard-Baron A (2007) Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med 33:444–447
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Etchecopar-Chevreuil, C., François, B., Clavel, M. et al. Cardiac morphological and functional changes during early septic shock: a transesophageal echocardiographic study. Intensive Care Med 34, 250–256 (2008). https://doi.org/10.1007/s00134-007-0929-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0929-z