Abstract
Objective
To evaluate the reliability of mini-bronchoalveolar lavage (mini-BAL) for the measurement of tobramycin concentrations in epithelial lining fluid (ELF) in comparison with conventional bronchoscopic bronchoalveolar lavage (BAL).
Design
Prospective, open-label study.
Setting
An intensive care unit and research ward in a university hospital.
Patients
Twelve critically ill adult patients with ventilator-associated pneumonia (VAP).
Interventions
All subjects received intravenous infusions of tobramycin 7–10 mg/kg once daily. After 2 days of therapy, the steady-state serum and ELF concentrations (obtained from BAL and mini-BAL) of tobramycin were determined by means of high-performance liquid chromatography.
Measurements and results
We observed poor penetration of tobramycin in ELF of ≈ 12% with ELF peak concentrations of ≈ 3 mg/l with both methods. Good agreement in Bland–Altman analysis (mean ± SD bias = 0.04 ± 0.38 mg/l) was observed between the two methods of sampling.
Conclusion
Our results suggest that tobramycin 7–10 mg/kg once daily in critically ill patients with VAP might provide insufficient lung concentrations in the case of difficult-to-treat pathogens. Besides, mini-BAL, which is simple, non-invasive and easily repeatable at the bedside, appears to be a reliable method for the measurement of antibiotic concentrations in ELF in comparison with bronchoscopic BAL in critically ill patients with VAP.
Similar content being viewed by others
Introduction
Although considerable knowledge has been recently gathered to improve the management of respiratory tract infections, ventilator-associated pneumonia (VAP) remains a frequent cause of death in critically ill patients [1, 2]. Some therapeutic failures may be related to drug-resistant pathogens but also to the inability of antibiotic agents to achieve adequate tissue concentrations at the site of infection [3, 4].
Tobramycin, usually in association with an antipseudomonal β-lactam, has been recently proposed in the American Thoracic Society Guidelines (ATS) as initial empiric therapy in patients with late-onset VAP or risk factors for multidrug-resistant pathogens [1].
The penetration of tobramycin in epithelial lining fluid (ELF), advocated as a reliable marker of extracellular concentrations [3, 4], has been studied in patients with pneumonia or in intensive care patients undergoing fiberoptic bronchoscopy for diagnostic purposes; however, no data are available concerning critically ill patients with VAP [5–7]. Moreover, many previous studies have reported the use of small-volume non-bronchoscopic bronchoalveolar lavage (“mini-BAL”) for measuring ELF antibiotic concentrations in critically ill patients with VAP, but the reliability of this method in comparison with conventional bronchoscopic BAL is unknown [8–12].
We thus conducted a study: (1) to determine the ELF concentrations of tobramycin obtained after BAL and mini-BAL sampling in critically ill patients with VAP; and (2) to determine the reliability of the mini-BAL method in comparison with the conventional BAL method.
Materials and methods
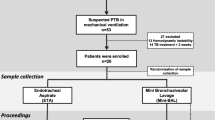
This was a prospective, open-label, single-center study approved by the local ethics committee. Prior to inclusion in the study, all patients or their closest relative provided written informed consent. Critically ill adult patients on mechanical ventilation were considered for inclusion when suspected of having late-onset VAP, i.e. clinical features of pneumonia with duration of ventilation of 5 days or more and/or antimicrobial therapy in the preceding 90 days [1, 2]. Patients were excluded from the study if they were allergic to tobramycin or exhibited renal dysfunction defined by a calculated creatinine clearance (using the urine of 24 h) of < 40 ml/min.
Before initiation of therapy, specimens for microbiologic diagnosis were obtained from all patients using a plugged telescoping catheter (Combicath®, Plastimed, St-Leu-La-Forêt, France), as previously described [13]. Tobramycin was administered as empirical therapy in association with an antipseudomonal β-lactam and vancomycin, as recommended in the ATS guidelines [1, 2]. Tobramycin was discontinued after 5 days of therapy and the total duration of antimicrobial therapy was 8–10 days, or 15 days in the case of VAP caused by non-fermentative Gram-negative bacilli [14].
All subjects received 30-min intravenous infusions of tobramycin 7 mg/kg once daily, as recommended, and the dose was adjusted (7–10 mg/kg) to obtain serum peak levels of 20–30 mg/l [1]. All samples for tobramycin concentration determinations were obtained at steady-state after 2 days of therapy. Blood samples were collected 30 min after the end of infusion and immediately centrifuged at 3,000 rpm for 5 min. The serum was removed and stored at –80 °C until analyzed. Simultaneously with blood sampling, all patients underwent standardized fibroscopic BAL sampling by infusing three 50-ml aliquots of sterile 0.9% saline solution [2]. The time elapsed between the beginning of BAL and the total recovery of the three aliquots did not exceed 2 min for each, to minimize free diffusion of urea through the alveolar epithelium, which may lead to falsely elevated concentrations of urea in the BAL fluid [3, 4]. Immediately after, each patient underwent mini-BAL procedure with 40 ml of sterile 0.9% normal saline solution, as previously described [8–12]. Both BAL and mini-BAL sampleswere immediately centrifuged at 3,000 rpm for 5 min and a single aliquot of supernatant was separated and frozen for the urea assay. The remaining volume was frozen at –80 °C until the assays were performed. All samples were assayed within 6 months from the time of their collection.
Concentrations of free tobramycin in serum and ELF were measured simultaneously after ultrafiltration through Microcon® filters (Millipore, France) by a liquid chromatography–mass spectrometry method [15]. The ultrafiltrate mean recoveries of tobramycin from quality control samples were 99% for serum and 98.5% for BAL. Detection was performed with a simple quad mass spectrometer equipped with an electrospray interface operated in positive mode. The assay was linear in the concentration range 0.05–50 μg/ml. The limit of quantification was 0.05 μg/ml and the assay precision was < 15% within and between batches.
As previously described, the concentration of free tobramycin in ELF (TBRELF) was determined as follows, using urea as an endogenous marker [16, 17]:
where TBRBAL is the measured concentration of free tobramycin in BAL or mini-BAL fluid, ureaSER is the concentration of urea in serum, and ureaBAL is the concentration of urea in BAL or mini-BAL fluid.
Bland–Altman analysis was performed with MedCalc® 8.2 software (Medisoftware, Mariakerke, Belgium) to evaluate the agreement between the two methods of ELF sampling [18].
Results
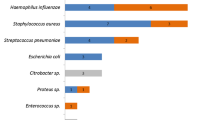
Twelve critically ill adult subjects with late-onset VAP completed the study (Table 1). Tobramycin administration, BAL and mini-BAL procedures were well tolerated and no adverse effects were observed. The mean ± SD steady-state peak serum and ELF tobramycin concentrations appear in Table 1. Bland–Altman analysis showed good agreement (mean ± SD bias = 0.04 ± 0.38 mg/l, with 95% confidence interval –0.2 to 0.3 mg/l) between the two methods (Fig. 1) [18]. Of the 12 patients undergoing tobramycin sampling, 8 had one organism recovered using the plugged telescoping catheter technique, all but one (Streptococcus pneumoniae) susceptible to tobramycin (2 P. aeruginosa, 2 oxacillin-resistant S. aureus, 3 Enterobacteriaceae and 1 S. pneumoniae). All patients but two (83%) had favorable outcome at the end of therapy. Two patients died from multiple-organ failure at days 3 and 5, respectively, with VAP caused by S. pneumoniae and P. aeruginosa.
Discussion
This is the first study to report ELF concentrations of free tobramycin in critically ill patients with VAP. Our results show a penetration of tobramycin in ELF of ≈ 12% with peak ELF concentrations of ≈ 3 mg/l. This percentage penetration is less than that reported in previous studies, where ELF/serum tobramycin concentration ratios of > 30% were observed [5–7]. However, these studies did not determine free tobramycin concentrations and were not performed in critically ill patients with VAP, which often presents pathophysiological conditions that may alter the pharmacokinetics of antimicrobial agents, explaining these differences.
As previously reported in patients with pneumonia, the peak ELF concentrations in the current study were lower than the susceptibility breakpoint (4 mg/l) for tobramycin [6]. Moreover, many experimental and clinical studies have shown that efficacy during the treatment of pneumonia with aminoglycosides was correlated with the ratio of peak concentration to minimum inhibitory concentration (C max/MIC), which should ideally exceed 10, and that one of the causes of treatment failure in VAP is incorrect antibiotic dosage [3, 4, 19]. Therefore, it appears that in critically ill patients with VAP, the use of tobramycin 7–10 mg/kg might provide insufficient ELF concentrations to optimize antimicrobial therapy with this agent. This suggests that in the case of VAP caused by difficult-to-treat pathogens with high MICs for tobramycin, higher doses should be administered in critically ill patients, with another agent used in combination.
The second aim of our study was to determine the reliability of the mini-BAL method in comparison with the conventional BAL method for the measurement of antibiotic concentrations in ELF. We found satisfactory results, with good agreement between the two methods of sampling. The mean difference between BAL and mini-BAL was 0.04 mg/l, with 95% confidence interval –0.2 to 0.3 mg/l; thus, mini-BAL tends to give lower values than conventional BAL, by between –0.2 and 0.3 mg/l. Despite this, the limits of agreement (–0.70 and 0.79 mg/l) are small enough for us to be confident that the mini-BAL method can be used in place of conventional BAL for the dosage of antibiotics in ELF. This is of importance since mini-BAL is simple, non-invasive and easily repeatable at the bedside, which might be advantageous in comparison with conventional bronchoscopic BAL.
Our study, however, presents some limitations. First, the mini-BAL procedure was always conducted after the BAL procedure; therefore, the slightly lower ratios obtained with the mini-BAL procedure may be the result of an experimental bias. Moreover, it has been shown that tobramycin distribution into lung is quite slow (from 30% at 30 min up to 153% at 8 h); therefore, the ≈ 12% penetration ratio observed in our study at one time point might have been higher at later times [6].
Conclusion
We have shown (1) that tobramycin exhibits ≈ 12% pulmonary diffusion at 30 min in critically ill patients in VAP, with ELF peak concentrations less than the susceptibility breakpoint, suggesting that a dose of 7–10 mg/kg daily might be insufficient for difficult-to-treat-pathogens; and (2) that mini-BAL is a reliable method of sampling for the measurement of antibiotics in ELF. Further studies evaluating the impact on outcome of higher doses of tobramycin are required to optimize the use of this antimicrobial agent in critically ill patients with VAP.
References
Niederman MS, Craven DE, Bonten MJ, Chastre J, Craig WA, Fagon JY, Hall J, Jacoby GA, Kollef MH, Luna CM, Mandell MA, Torres A, Wunderink RG (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416
Chastre J, Fagon JY (2002) Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903
Cazzola M, Blasi F, Terzano C, Matera MG, Marsico SA (2002) Delivering antibacterials to the lungs: considerations for optimizing outcomes. Am J Respir Med 1:261–272
Chiu LM, Amsden GW (2002) Intrapulmonary pharmacokinetics of antibacterial agents: implications for therapeutics. Am J Respir Med 1:201–209
Braude AC, Hornstein A, Klein M, Vas S, Rebuck AS (1983) Pulmonary disposition of tobramycin. Am Rev Respir Dis 127:563–565
Carcas AJ, Garcia-Satue JL, Zapater P, Frias-Iniesta J (1999) Tobramycin penetration into epithelial lining fluid of patients with pneumonia. Clin Pharmacol Ther 65:245–250
Mazzei T, Novelli A, De Lalla F, Mini E, Periti P (1995) Tissue penetration and pulmonary disposition of tobramycin. J Chemother 7:363–370
Boselli E, Breilh D, Cannesson M, Xuereb F, Rimmelé T, Chassard D, Saux MC, Allaouchiche B (2004) Steady-state plasma and intrapulmonary concentrations of piperacillin/tazobactam 4 g/0.5 g administered to critically ill patients with severe nosocomial pneumonia. Intensive Care Med 30:976–979
Boselli E, Breilh D, Duflo F, Saux MC, Debon R, Chassard D, Allaouchiche B (2003) Steady-state plasma and intrapulmonary concentrations of cefepime administered in continuous infusion in critically ill patients with severe nosocomial pneumonia. Crit Care Med 31:2102–2106
Boselli E, Breilh D, Rimmelé T, Djabarouti S, Saux MC, Chassard D, Allaouchiche B (2005) Pharmacokinetics and intrapulmonary diffusion of levofloxacin in critically ill patients with severe community-acquired pneumonia. Crit Care Med 33:104–109
Boselli E, Breilh D, Rimmelé T, Djabarouti S, Toutain J, Chassard D, Saux MC, Allaouchiche B (2005) Pharmacokinetics and intrapulmonary concentrations of linezolid administered to critically ill patients with ventilator-associated pneumonia. Crit Care Med 33:1529–1533
Boselli E, Breilh D, Rimmelé T, Poupelin JC, Saux MC, Chassard D, Allaouchiche B (2004) Plasma and lung concentrations of ceftazidime administered in continuous infusion to critically ill patients with severe nosocomial pneumonia. Intensive Care Med 30:989–991
Pham LH, Brun-Buisson C, Legrand P, Rauss A, Verra F, Brochard L, Lemaire F (1991) Diagnosis of nosocomial pneumonia in mechanically ventilated patients. Comparison of a plugged telescoping catheter with the protected specimen brush. Am Rev Respir Dis 143:1055–1061
Chastre J, Wolff M, Fagon JY, Chevret S, Thomas F, Wermert D, Clementi E, Gonzalez J, Jusserand D, Asfar P, Perrin D, Fieux F, Aubas S (2003) Comparison of 8 vs. 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA 290:2588–2598
Keevil BG, Lockhart SJ, Cooper DP (2003) Determination of tobramycin in serum using liquid chromatography–tandem mass spectrometry and comparison with a fluorescence polarisation assay. J Chromatogr B Analyt Technol Biomed Life Sci 794:329–335
Dargaville PA, South M, Vervaart P, McDougall PN (1999) Validity of markers of dilution in small volume lung lavage. Am J Respir Crit Care Med 160:778–784
Yamazaki K, Ogura S, Ishizaka A, Oh-hara T, Nishimura M (2003) Bronchoscopic microsampling method for measuring drug concentration in epithelial lining fluid. Am J Respir Crit Care Med 168:1304–1307
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Kashuba AD, Nafziger AN, Drusano GL, Bertino JS Jr (1999) Optimizing aminoglycoside therapy for nosocomial pneumonia caused by Gram-negative bacteria. Antimicrob Agents Chemother 43:623–629
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: http://dx.doi.org/10.1007/s00134-007-0689-9.
Support was provided only by institutional sources.
Rights and permissions
About this article
Cite this article
Boselli, E., Breilh, D., Djabarouti, S. et al. Reliability of mini-bronchoalveolar lavage for the measurement of epithelial lining fluid concentrations of tobramycin in critically ill patients. Intensive Care Med 33, 1519–1523 (2007). https://doi.org/10.1007/s00134-007-0688-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0688-x