Abstract
Objective
To determine the steady-state plasma and epithelial lining fluid (ELF) concentrations of ceftazidime administered in continuous infusion to critically ill patients with severe nosocomial pneumonia.
Design
Prospective, open-label study.
Setting
An intensive care unit and research ward in a university hospital.
Patients
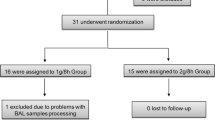
A total of 15 adult patients with severe nosocomial bacterial pneumonia on mechanical ventilation were enrolled.
Interventions
All subjects received a 30 min intravenous infusion of 2 g ceftazidime followed by a continuous infusion of 4 g over 24 h. The concentrations of ceftazidime in plasma and ELF were determined at steady-state after 2 days of therapy by high performance liquid chromatography.
Measurements and main results
The mean ±SD steady-state plasma and ELF concentrations of 4 g ceftazidime in continuous infusion were 39.6±15.2 µg/mL and 8.2±4.8 µg/mL, respectively, showing a mean ±SD percentage penetration of ceftazidime into ELF of 20.6±8.9%.
Conclusion
The administration of 4 g ceftazidime in continuous infusion in critically ill patients with severe nosocomial pneumonia provides concentrations in excess of the minimal inhibitory concentration of many susceptible organisms over the course of therapy both in serum and ELF. However, for some pathogens such as P. aeruginosa, higher doses of ceftazidime should be administered, or another agent should be used in combination.
Similar content being viewed by others
Introduction
Ceftazidime is a β-lactam antibiotic widely used in the treatment of severe infections generally caused by nosocomial Gram-negative pathogens. Due to its wide spectrum, including Pseudomonas aeruginosa, ceftazidime has become a major agent in the treatment of nosocomial pneumonia.
It is generally accepted today that one important determinant for the efficacy of β-lactams is the time the drug concentration is above the minimal inhibitory concentration (MIC) for the pathogen during the dosing interval [1]. Therefore, the administration by continuous infusion appears to optimize the pharmacodynamic profile of β-lactam antibiotics by providing concentrations in excess of the MIC of the pathogens during the time course of therapy [1].
The appropriate antibiotic therapy of pneumonia requires achievement of significant concentrations of antibiotics at the site of infection [2]. Epithelial lining fluid (ELF) has been advocated as an important infection site for common extracellular pathogens in lung tissue, and the measure of the concentration of antibiotics in ELF is considered as a reliable marker of the concentration of antibiotics into lung tissue [2, 3]. Thus far, a limited number of studies have evaluated the penetration of ceftazidime into lung tissue, but the dosages were generally not performed in ELF, and the antibiotic was not administered in continuous infusion [3].
The purpose of this study was to determine the steady-state plasma and ELF concentrations of ceftazidime administered in continuous infusion to critically ill patients with severe nosocomial bacterial pneumonia.
Subjects and methods
Study design and subjects
This was a prospective, open-label, single-center study approved by the local ethics committee, performed between July 2001 and April 2002. Prior to inclusion in the study, all patients or their closest relatives provided written informed consent. Critically ill adult patients who were hospitalized in intensive care unit for at least 72 h prior to diagnosis were considered eligible for inclusion in the study when suspected of having severe nosocomial pneumonia, i.e. requiring mechanical ventilation.
The patients were excluded from the study if they were allergic to ß-lactam antibiotics, received antibiotics prior to the study, exhibited renal dysfunction defined by a calculated creatinine clearance (using the urine over 24 h) of <40 mL/min or a serum creatinine concentration of >200 µmol/L, or had impairment of hepatic function (alanine aminotransferase, aspartate aminotransferase or bilirubin greater than twice the upper normal limit).
Before initiation of therapy, specimens for microbiologic diagnosis were obtained using a plugged telescoping catheter (Combicath, Plastimed, St-Leu-La-Forêt, France) from all the patients [4]. All patients were on sedation and mechanical ventilation during the procedure, which is simple, non-invasive and easily repeatable at the bedside. Ceftazidime was then administered as empirical therapy in addition to amikacin, which is the standard protocol in our institution, until identification of the pathogen and determination of its antibiotic susceptibility. No vancomycin was added, as no patient received antibiotics prior to the study.
All subjects received a 30 min intravenous infusion of 2 g ceftazidime followed by a continuous infusion of 4 g over 24 . All samples for ceftazidime concentration determinations were obtained at steady-state after 2 days of therapy. Blood samples were collected at 3 predetermined time points at 8:00 a.m., 12:00 p.m. and 6:00 p.m. and were immediately centrifuged at 3,000 rpm for 5 min. The serum was removed and stored at −20°C until analyzed. Each subject underwent simultaneously to blood sampling one standardized bronchoalveolar microlavage (BAL) procedure, as previously described [5]. A standard bronchial brush tube (Combicath, Plastimed, St-Leu-La-Forêt, France) was inserted in the endotracheal tube, and used to perform a mini-BAL with 40 mL of sterile 0.9% normal saline solution. The aspirate was immediately centrifuged at 3,000 rpm for 5 min and a single aliquot of supernatant was separated and frozen for the urea assay. The remaining volume was frozen at −20°C until the assays were performed. All blood and BAL samples were assayed within 6 months from the time of collection.
Drug and urea assays
The concentrations of ceftazidime in plasma and BAL were determined by high performance liquid chromatography (HPLC). The detection chosen for the HPLC assay was an ultraviolet detection set at a wavelength of 263 nm. Cefsulodine was used as internal standard. The extraction recoveries of ceftazidime from quality control samples were 91% and 95% and 96% and 90%, respectively for plasma and BAL. Intraday and interday coefficients of variation of ceftazidime were <5% both for plasma and BAL samples. The limit of detection and the limit of quantification of ceftazidime were 0.02 µg/mL and 0.08 µg/mL, respectively, for both plasma and BAL samples.
Calculation of ceftazidime concentrations in ELF
As previously described, the concentration of ceftazidime in ELF (CAZELF) was determined as follows, with urea as an endogenous marker [5]:
where CAZBAL is the measured concentration of ceftazidime in BAL fluid, ureaBAL is the concentration of urea in the BAL fluid and ureaSER is the concentration of urea in plasma.
Results
A total of 15 adult subjects (9 men and 6 women, mean age ±SD 57±14 years, weight 70±11 Kg and creatinine clearance 54±37 mL/min) with nosocomial ventilator-associated pneumonia, completed the study. Ceftazidime and microlavage procedures were well tolerated and no serious adverse effects were observed. In total, 14 pathogens were isolated in this study population (5 Enterobacteriaceae, 4 Pseudomonas aeruginosa, 3 Staphylococcus aureus, 1 Acinetobacter baumanii and 1 Streptococcus pneumoniae). After determination of the causative pathogen susceptibility (after at least 48 h of therapy), the narrowest spectrum antibiotic combination was administered whenever possible.
The individual steady-state serum and ELF concentrations of ceftazidime are shown in Fig. 1. The mean ±SD concentrations of 4 g ceftazidime over 24 h in continuous infusion were 39.6±15.2 µg/mL in plasma and 8.2±4.8 µg/mL in ELF, showing a mean ±SD penetration of ceftazidime into ELF of 20.6±8.9%.
Individual steady-state serum (filled circles) and ELF (open circles) concentrations of continuous infusion of 4 g ceftazidime administered to critically ill patients with severe bacterial pneumonia (ELF epithelial lining fluid). The dotted line represents the susceptibility breakpoint (8 µg/mL) for ceftazidime [10]
Discussion
It has been shown that one major pharmacodynamic parameter to predict the efficacy of β-lactam antibiotics is the time above the MIC (T>MIC), which is used as an argument to administer these antimicrobial agents by continuous infusion [6]. Although clinical studies comparing the efficacy of intermittent and continuous infusion of β-lactam antibiotics in humans are scarce, studies in vitro and in laboratory animals generally show continuous infusion to be more efficacious [1].
Pharmacokinetics and pharmacodynamics of continuous infusion of ceftazidime have been extensively studied in various in vitro and human models [7, 8]. These studies suggest that the administration of ceftazidime in continuous infusion appears to optimize the pharmacodynamic profile of this agent by providing concentrations in excess of the MIC of most of the causative pathogens. Furthermore, data related to ceftazidime indicate that the drug concentration at steady-state should exceed 1–4 times the pathogen MIC [1]. It has been shown for P. aeruginosa in an in vitro pharmacokinetic model that a sustained level of ceftazidime around or slightly above the MIC was not high enough to maintain efficacy and that sustained concentrations exceeding 4 times the MIC were required [7].
Considering the targeted micro-organisms commonly encountered in ventilator-associated pneumonia [9] and the reported range of these pathogens MIC values for ceftazidime in nosocomial infections (P. aeruginosa, 4–8 µg/mL, S. aureus, 3–4 µg/mL, Enterobacteriaceae, 0.38–>256 µg/mL and A. baumanii, 8–32 µg/mL), it appears that a regimen of 4 g ceftazidime over 24 h might provide insufficient concentrations into ELF to achieve an optimal T>MIC. Moreover, sustained concentrations higher than 4 times the causative pathogen MIC might not be achieved with that regimen in the treatment of severe nosocomial pneumonia, which might lead to a selection of resistant strains. This suggests that during the treatment of nosocomial pneumonia caused by pathogens with high MICs for ceftazidime, higher doses than 4 g ceftazidime should be administered, or that a second agent should be used in combination.
Conclusion
In conclusion, the administration of ceftazidime in continuous infusion provides concentrations in excess of the MIC of many susceptible organisms over the course of therapy both in serum and ELF. However, during the treatment of nosocomial pneumonia caused by pathogens with potentially high MICs such as P. aeruginosa, higher doses than 4 g continuous ceftazidime should be administered to maintain a T>MIC of 100% with a concentration into ELF higher than 4 times the MIC, or another agent should be used in combination.
Further studies comparing the outcomes of critically ill patients with severe pneumonia caused by nosocomial pathogens with high MICs and treated with continuous or intermittent infusion of ceftazidime are needed to determine whether the optimization of the pharmacodynamic profile of this agent provides an effective clinical benefit.
References
MacGowan AP, Bowker KE (1998) Continuous infusion of β-lactam antibiotics. Clin Pharmacokinet 35:391–402
Bergogne-Berezin E (1995) New concepts in the pulmonary disposition of antibiotics. Pulm Pharmacol 8:65–81
Boselli E, Allaouchiche B (2001) Diffusion pulmonaire des antibiotiques. Analyse critique de la littérature. Ann Fr Anesth Réanim 20:612–630
Pham LH, Brun-Buisson C, Legrand P, Rauss A, Verra F, Brochard L, Lemaire F (1991) Diagnosis of nosocomial pneumonia in mechanically ventilated patients. Comparison of a plugged telescoping catheter with the protected specimen brush. Am Rev Respir Dis 143:1055–1061
Boselli E, Breilh D, Duflo F, Saux MC, Debon R, Chassard D, Allaouchiche B (2003) Steady-state plasma and intrapulmonary concentrations of cefepime administered in continuous infusion in critically ill patients with severe nosocomial pneumonia. Crit Care Med 31:2102–2106
Mouton JW, Vinks AA (1996) Is continuous infusion of beta-lactam antibiotics worthwhile?—Efficacy and pharmacokinetic considerations. J Antimicrob Chemother 38:5–15
Mouton JW, den Hollander JG (1994) Killing of Pseudomonas aeruginosa during continuous and intermittent infusion of ceftazidime in an in vitro pharmacokinetic model. Antimicrob Agents Chemother 38:931–936
Nicolau DP, MacNabb JC, Lacy MK, Li J, Quintiliani R, Nightingale CH (1999) Pharmacokinetics and pharmacodynamics of continuous and intermittent ceftazidime during the treatment of nosocomial pneumonia. Clin Drug Invest 18:133–139
Chastre J, Fagon JY (2002) Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903
Hanberger H, Nilsson LE, Claesson B et al. (1999) New species-related MIC breakpoints for early detection of development of resistance among Gram-negative bacteria in Swedish intensive care units. J Antimicrob Chemother 44:611–619
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boselli, E., Breilh, D., Rimmelé, T. et al. Plasma and lung concentrations of ceftazidime administered in continuous infusion to critically ill patients with severe nosocomial pneumonia. Intensive Care Med 30, 989–991 (2004). https://doi.org/10.1007/s00134-004-2171-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2171-2