Abstract
Objective
To evaluate a new approach for monitoring and improving patient-ventilator interaction that utilizes a signal generated by the equation of motion, using improvised values for resistance and elastance obtained noninvasively.
Design and setting
Observational study in intensive care units in five European centers.
Patients
We studied 21 stable patients instrumented with esophageal/gastric catheters for a previous study and ventilated alternately with pressure support (PSV) and proportional assist (PAV) ventilation with a Tyco 840 ventilator.
Measurements and results
Previously recorded digital files were analyzed in real-time by a prototype incorporating the new technology (PVI monitor, YRT, Winnipeg, Canada). Actual onsets (PDI-TONSET) and ends (PDI-TEND) of inspiratory efforts, ineffective efforts, and patient respiratory rate were identified visually from transdiaphragmatic or calculated respiratory muscle pressure. Monitor-identified TONSET occurred 0.107 ± 0.074 s after PDI-TONSET, substantially less than trigger delay observed with conventional triggering (0.326 ± 0.086 s). End of effort was identified 0.097 ± 0.096 s after PDI-TEND, significantly less than actual cycling-off delay during PSV (0.486 ± 0.307 s) or PAV (0.277 ± 0.084 s). The monitor detected 80% of ineffective efforts. There was excellent agreement between monitor-estimated respiratory rate and actual patient rate over a wide range (17–59/min) of patient rates (mean ( ± SD) of difference −0.2 ± 1.9/min for pressure support and 0.2 ± 0.9/min for proportional assist) even when large discrepancies existed (> 35/min) between patient and ventilator rates.
Conclusions
The proposed approach should make it possible to improve patient-ventilator interaction and to obtain accurate estimates of true patient respiratory rate when there is nonsynchrony.
Similar content being viewed by others
Introduction
With conventional pressure or flow triggering, when dynamic hyperinflation is present, triggering does not occur until the patient makes enough effort to overcome the elastic recoil of the respiratory system present at the onset of effort [1–3]. Because dynamic hyperinflation is very frequent in ventilated patients [1, 2, 4], triggering is often substantially delayed. In many cases the effort terminates before the ventilator is triggered (ineffective efforts [1, 4, 5]). With pressure support and volume-cycled ventilation the ventilator cycle is not always constrained to cycle-off at the end of inspiratory effort [3]. As a result ventilator cycle may terminate before the end of effort or extend well into the patient's expiratory phase [3, 5]. There are reasons to believe that nonsynchrony may have clinical consequences [6, 7]. Asynchrony may result in increased need for sedation, impairment of sleep quality, or respiratory muscle injury (see [7] for review). Nonsynchrony may also lead to false diagnosis of weaning failure, with unnecessary continuation of mechanical ventilation [5, 7]. These consequences may underlie the recent demonstration that the duration of mechanical ventilation is longer in patients with nonsynchrony [6].
An entirely different approach to triggering and cycling-off has recently been proposed [8] in which the equation of motion [9, 10] is used to generate in real-time a signal that reflects respiratory muscle pressure output. However, instead of requiring real values of passive elastance and resistance, which are difficult to obtain during assisted ventilation, this approach utilizes improvised values that are selected empirically (see the Electronic Supplementary Material, ESM). This study compared the triggering and cycling-off times observed during conventional triggering with times determined from the improvised effort signal (PSIGNAL).
Methods
A prototype (PVI Monitor, YRT, Winnipeg, Canada) was tested on 21 preexisting files that contained periods on pressure support (PSV, n = 20) and proportional assist ventilation (PAV; n = 21). These were generated during an earlier unrelated study involving patients with acute respiratory failure in stable condition [11]. The ethics committees of participating institutions approved the original clinical study. Patients or next-of-kin gave informed consent (see detailed methods in ESM).
Files contained airway pressure (Paw), flow (\( \dot{\text{V}}\)), integrated flow (V), esophageal pressure (PESO), and, in 14 patients, gastric pressure (PGA). Diaphragm pressure (PDI = PGA−PESO) or, when PGA was unavailable, respiratory muscle pressure (PMUS; according to the Campbell method [12]) was calculated (see ESM). Approximately 1 hour from each file was analyzed. Markers were placed at the beginning (PDI-TONSET) and end (PDI-TEND) of each effort (Fig. 1). In four patients PDI-TEND could not be identified (see ESM). Respiratory mechanics were calculated using standard methods. Flow at PDI-TONSET was measured (flow at end-expiration). Negative values indicated the presence of intrinsic PEEP (PEEPI). When PDI was available (n = 14), the increase in PDI at zero flow crossing was measured to provide the component of PEEPI related to dynamic hyperinflation (PEEPI(DH) [13]). Monitor processed Paw and flow data (at 200 Hz) as if received in real-time, generated a PSIGNAL in real-time and made the following identifications (Fig. 1):
-
Real-time TONSET was identified when PSIGNAL increased by a threshold amount. TONSET threshold is automatically adjusted based on baseline noise in the 20 most recent breaths.
-
Real-time TEND was identified when PSIGNAL declined by 50% of the increase measured since real-time TONSET.
-
Retrospective TONSET was determined, after breaths had elapsed, as the time when PSIGNALbegan rising (as opposed to real-time TONSET where PSIGNAL must exceed a threshold to avoid autotriggering; Fig. 1). This is intended for noninvasive monitoring of trigger delay.
-
Retrospective TEND was identified retrospectively as the point where PSIGNALbegan declining (cf. real-time TEND where PSIGNAL must decline a threshold amount to avoid false cycling-off).
-
Ineffective efforts were identified retrospectively when an increase in PSIGNAL met minimum criteria for efforts but were not associated with a new ventilator breath. Ineffective efforts occurring during the inflation phase are referred to as extra-efforts.
-
Patient's respiratory rate was the sum/minute of TONSETS that triggered ventilator breaths and ineffective/extra-efforts.
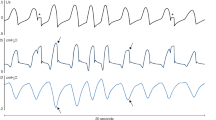
Example of monitor output (top three channels) and the corresponding diaphragm pressure (PDI). PAW Airway pressure; PSIGNAL signal generated by monitor in real-time; TTRIGGER time of ventilator triggering. Timing events identified by monitor are indicated in the PSIGNAL tracing whereas the corresponding actual timing events are indicated in the PDI tracing. PDI-TONSET and PDI-TEND are onset and end of effort as scored manually by an observer; Real-time TONSET and real-time TEND are onset and end of efforts identified by monitor in real-time; Retro TONSET and Retro TEND are onset and end of efforts identified by monitor retrospectively (i. e., after breath had elapsed). See text
Sensitivity in detecting efforts was determined from the proportion PDI/PMUS efforts detected as efforts by monitor. False efforts were defined as efforts identified by the monitor that had no corresponding PDI/PMUS effort. Respiratory rates computed by monitor and from PDI/PMUS were compared by paired t-test. Actual trigger delay (trigger time minus PDI-TONSET; Fig. 1) and cycling-off delay (cycling-off time minus PDI-TEND) were calculated for each triggered breath. The corresponding values if monitor's real-time TONSET and TEND were used instead for triggering and cycling-off were also calculated. Pressure-time product required for conventional triggering (PTPTRIG) and if real-time TONSET were used instead for triggering [PTPTRIG(monitor)] were calculated (see ESM). Ventilator TI was expressed as a fraction of patient TTOT (i. e., 60/patient respiratory rate). All reported values are mean ± SD.
Results
Physiological characteristics of the patients
Respiratory mechanics varied widely (Table S1, ESM). Four patients had severe expiratory flow limitation. Most patients had intrinsic PEEP (flow at end-expiration −0.235 ± 0.176 l/s; Table S1, ESM). PEEPI due to DH was 3.6 ± 2.6 cmH2O. There were no significant differences on average between PSV and PAV in PEEP level (6.5 ± 4.1 vs. 6.7 ± 3.9 cmH2O), pressure assist (peak PAW-PEEP; 17.7 ± 4.0 vs. 16.6 ± 4.6 cmH2O), or minute ventilation (11.4 ± 4.2 vs. 11.6 ± 3.8 l/min). Amplitude of PDI/PMUS excursions was relatively low (6.8 ± 4.1 vs. 8.8 ± 4.0 cmH2O for PSV and PAV; p < 0.01). Diaphragm rate was high on average (30.6 ± 8.4 vs. 30.8 ± 9.5/min for PSV and PAV, NS). Inspiratory duration of diaphragm efforts TI(DIA) was brief in both modes (0.66 ± 0.17 vs. 0.69 ± 0.14 s; NS).
Trigger-delay
Trigger-delay was 0.33 ± 0.09 and 0.32 ± 0.08 s in PSV and PAV, representing 51 ± 16% and 48 ± 12% of TI(DIA) (NS). PTPTRIG was 0.96 ± 0.75 and 1.06 ± 0.74 cmH2O·s for PSV and PAV (NS). Individual values are shown in Fig. 2 (PSV and PAV data were combined). Retrospective TONSET was identified ahead of triggering in 99.9 ± 0.2% of patient-triggered breaths (Table 1). It occurred slightly later than PDI-TONSET (dT = 0.059 ± 0.057 s; Table 1). dT was longer in patients with severe flow limitation in whom inspiratory effort often progresses for sometime before there is any external change (e. g., in flow) that can be measured by the monitor (e. g., Fig. S2, ESM). In patients without flow limitation dT was 0.042 ± 0.040 s.
Impact of using monitor's real-time TONSET on trigger delay (a) and on pressure time product preceding triggering (PTPTRIG; b). Conv. Trig Conventional flow triggering on the 840 ventilator; horizontal bars averages for patients with (dashed lines) and without severe flow limitation; *p < 0.02; **p < 0.001; ***p < 0.0001 lower than with conventional triggering
Real-time TONSET occurred ahead of conventional triggering in 95.9% of ventilator breaths (Table 1). Of the missed events 81% were when inspiratory effort began very early in the ventilator's exhalation phase and these efforts were deliberately blanked to avoid premature triggering (see ESM). The reason for the remaining missed TONSET instances (approx. 1% of breaths) was usually a high threshold related to baseline noise (e. g., cardiac artifacts, secretions).
The time difference between PDI-TONSET and real-time TONSET (DTONSET; what trigger-delay would be if real-time TONSET were used for triggering under identical conditions) averaged 0.107 ± 0.074 s (Fig. 2, Table 1). It was longer in patients with flow-limitation (0.21 ± 0.07 vs. 0.08 ± 0.05 s; Fig. 2). Concurrently PTPTRIG decreased from 0.92 ± 0.65 to 0.09 ± 0.1 cmH2O·s (p < 0.0001) and from 1.63 ± 0.95 to 0.51 ± 0.37 cmH2O·s (p < 0.02) in patients without and with flow-limitation (Fig. 2B). False TONSET instances, which would result in autotriggering, averaged 0.72/min, not exceeding 2.5/min in any patient (Table 1). Actual autotriggering (ventilator breaths without a PDI effort) averaged 0.23/min. The pattern of autotriggering was quite different in the two cases. Actual autotriggering occurred almost exclusively in three patients on PSV when the assist was excessive and efforts were indistinguishable from cardiac artifacts (e. g., Fig. S3, ESM). By contrast, monitor-generated false TONSET instances were sporadic and occurred in virtually all patients (Table 1).
Cycling-off delay
In PSV the cycling-off delay varied widely from 0.09 to 1.21 s (Fig. 3), representing 18–186% (47 ± 46%) of patient TE. With PAV the cycling-off delay ranged from 0.17 to 0.51 (Fig. 3) not exceeding 38% of TE(DIA) in any patient. The ratio of ventilator TI to patient TTOT ranged from 0.20 to1.27 in PSV while always remaining in the physiological range in PAV (0.20–0.44). Interestingly, ineffective efforts appeared in any patient in whom this ratio was greater than 0.42. In 13 of 17 patients in whom PDITEND was available retrospective TEND was within ± 0.1 s of the corresponding PDITEND (Table 1). In four patients it preceded PDI-TEND by more than 0.1 s. Real-time TEND occurred 0.097 ± 0.096 s after PDITEND and was identified before PDI-TEND in three patients (Table 1). Figure 3 shows how cycling-off delay would be affected if real-time TEND were used for cycling-off the ventilator under identical conditions.
Impact of ventilator settings on monitor's identification of TONSET and TEND
Table S2 (ESM)) shows individual values of DTONSET and DTEND along with PAW, VT, and PDI/PMUS. Ventilator settings, VT, and PDI varied substantially between patients. Notably, PDI ranged from 1.5 to 16.5 cmH2O. However, there was no correlation between PAW, tidal volume, or PDI and either DTONSET or DTEND with either PSV or PAV. Likewise, although on average PAW, VT and PDI did not differ substantially between PSV and PAV, within-patient differences were large in some cases (Table S2, ESM). The differences in DTONSET between PSV and PAV were small (0.007 ± 0.025 s) and were not correlated with differences in PAW, VT or PDI, indicating that they were not related to ventilator settings. Likewise, the differences in DTEND between PSV and PAV were small (0.001 ± 0.065 s) and not correlated with differences in PAW, VT or PDI.
Ineffective/extra-efforts
In seven patients ineffective/extra-efforts were frequent (> 10% of RR) in PSV and resulted in an important difference between patient and ventilator rates (up to 32/min, open circles, Fig. 4 left). In the same patients on PAV ineffective efforts were either rare (n = 3) or considerably fewer (Fig. 4 right). A total of 2,893 ineffective/extra-efforts were identified in the PDI tracing (Table 1). The monitor identified 2,319 of these (79.7%). Two-thirds of the unidentified efforts occurred in two patients with severe flow limitation (nos. 14, 19; Table 1) in whom these efforts had virtually no effect on flow (e. g., Fig. S2, ESM). In most other cases the unidentified events were associated with very weak PDI efforts. False identification of ineffective efforts occurred infrequently (0.2 ± 0.5/min), with the highest rate being 2.1/min (Table 1).
Solid symbols Relationship between respiratory rate identified by monitor (RRMONITOR) and respiratory rate identified from diaphragm pressure (RRPDI) during pressure support (PSV, n = 20 patients) and proportional assist ventilation (PAV, n = 21 patients). Note the excellent agreement between the two measurements in both modes. Open symbols Relationship between ventilator rate (RRVENT) and RRPDI. This relationship is quite poor in PSV
Patient's respiratory rate
Solid circles in Fig. 4 show the relationship between respiratory rate estimated by monitor and that from PDI. The agreement was excellent both in PSV and PAV, with an average difference of −0.2 ± 1.9/min in PSV and 0.2 ± 0.9/min in PAV. By contrast, the relationship between ventilator rate and PDI rate (open circles) was quite poor in PSV (r = 0.43, NS).
Improvised elastance (KV) and resistance (KF) values
Table S3 (ESM) shows individual values of KV and KF and their within-patient variability during PSV and PAV. Average KV ranged from 12.2 to 43.6 in PSV (27.8 ± 9.1) and from 12.9 to 40.5 in PAV (25.2 ± 7.6; p < 0.05). KF ranged from 2.5 to 24.1 in PSV (11.4 ± 6.3) and from 2.3 to 21.0 in PAV (10.2 ± 5.8) (p < 0.005). KV and KF changed from time to time in each patient but the changes were relatively small (Table S3, ESM). Figure S5 (ESM) shows the relation between KV and KF (average of PSV and PAV values) and the corresponding ERS and RLUNG measured by use of esophageal pressure. There was a significant correlation in both cases (p < 0.0001) but there was considerable scatter. Average difference ( ± SD) was −4.8 ± 6.1 cmH2O/l for KV vs. ERS and 1.5 ± 4.1 cmH2O/l per second for KF vs. RLUNG.
Discussion
The current study shows that a signal generated noninvasively from flow, volume, and PAW using improvised resistance and elastance values can be used for monitoring patient-ventilator interaction and for providing information to optimize ventilator settings. The signal can also potentially be used to directly synchronize onsets and ends of ventilator cycles with patient efforts.
Critique of methods
We used preexisting files to test this approach. However, data were inputted in the monitor's computer in exactly the same fashion as would happen if they were acquired live. Because there were no interventions, results would have been the same if the patients were monitored live. It may be argued that using 15% of peak flow for cycling-off PSV may have unduly exaggerated the nonsynchrony in this mode. Using simulations, however, we found that the magnitude of nonsynchrony is only marginally greater with 15% peak than with the currently used 25%. Furthermore, extent of nonsynchrony found here is not substantially different from what was reported previously with PSV [1, 2, 4, 7].
This study showed that for identical ventilator outputs (PAW and flow) and patient outputs (PDI, patient RR) TONSET, and TEND are closer to the true onsets and ends of the patient's efforts than conventional triggering algorithms (Figs. 2, 3). However, TONSET and TEND did not actively control ventilator cycles. It is possible that if this approach were used to directly control the ventilator's timing a number of variables that affect synchrony may have changed (e. g., VE, PMUS, dynamic hyperinflation [1, 2, 5, 14]), so that the net effect on triggering and cycling-off delays may have differed from that reported here. However, TONSET and TEND were superior to conventional triggering in all patients (Figs. 2, 3), and in the same patients under two different modes, despite large differences in ventilator and patient outputs (Table S2, ESM). This suggests that the current approach remains superior to conventional triggering regardless of how the patient and ventilator respond to the different triggering method. The current approach was validated in intubated patients, in whom leaks are minimal, and externally measured flow may be assumed to reflect flow in and out of the patient with reasonable accuracy. The extent to which the accuracy of this method is influenced by leaks, such as may exist during noninvasive ventilation, is not known.
Use of PSIGNAL for active triggering and cycling-off
Real-time TONSET occurred before conventional triggering in 95.9% of triggered breaths (Table 1). Most of the missed TONSET instances were deliberately blocked because the associated efforts occurred too early in the ventilator's expiratory phase (due to excessive cycling-off delay). Since the current system would preclude excessive cycling-off delay, instances where an effort would be missed for this reason would also be reduced. Thus this approach can reduce trigger delay in virtually every breath. Figure 3 shows that the potential reduction in trigger-delay is substantial.
In general, improved trigger sensitivity is associated with increased likelihood for autotriggering (e. g., [4]). This was not the case here; frequency of false TONSET instances remained quite low in all patients (Table 1). More importantly, the autotriggering that may occur would not result in overventilation, the main side effect of autotriggering [15, 16]. With the current approach autotriggering can only be sporadic. A false TONSET can be identified as false retrospectively because the change in PSIGNAL would not meet respiratory effort criteria. TONSET threshold is then automatically increased thereby aborting further false triggers until the noise disappears. By contrast, with conventional triggering it is impossible to determine whether triggering was false in order to effect automatic changes in triggering threshold. Thus autotriggering may continue indefinitely [15], as was observed in three patients here (e. g., Fig. S3, ESM).
Real-time TEND occurred when PSIGNAL decreased to 50% of its peak value (Fig. 1). Is this appropriate? During unassisted breathing the onset of expiratory flow is invariably delayed relative to end of neural inspiration [10, 17, 18]. This delay is a function of rate of decline in PMUS and of respiratory mechanics (resistance × compliance, RC) [17]. Because these vary greatly between individuals, there is no unique optimal cycling-off delay (see Fig. S4, ESM). Ideally cycling-off should be linked to the rate of decline in PMUS and to RC. In the current prototype real-time TEND was linked to the rate of decline in PSIGNAL since with a faster declining rate the 50% point would be reached sooner. The choice of 50% was a compromise consistent with an average RC of approx. 0.6 s (R = 15, C = 0.04 [19, 20]). It would be possible to link the threshold proportional reduction to KV and KF, but at present the relationship between these values and actual respiratory mechanics is not sufficiently strong (Fig. S5, ESM).
Although the cycling-off delay that would result from using real-time TEND is appropriate on average (Fig. 3), it would not be prudent to rely entirely on real-time TEND. First, in three patients real-time TEND occurred before the end of patient TI (Table 1). This is likely related to nonlinearities in the pressure-flow and/or pressure-volume relationships. Second, in some patients on PSV inspiratory effort ends immediately after triggering [21, 22, 23] so that cycling-off the ventilator at the end of effort would result in very short ventilator TI. Accordingly, it would be necessary to establish a minimum duration for ventilator TI.
Prinianakis et al. [4] evaluated a new triggering/cycling-off algorithm for PSV based on shape of the flow waveform. Relative to conventional flow-triggering, there was a modest reduction in trigger-delay (approx. 0.04 s) and in PTPTRIG (approx. 0.1 cmH2O·s; see Fig. 2). There were no changes in ventilator TI, suggesting that cycling-off delay was not reduced. Ineffective efforts decreased marginally. For example, in the patient with the most ineffective efforts they decreased from 32 to 28/min. Although improvement in triggering was modest, autotriggering increased significantly [4].
Use of PSIGNAL for passive monitoring of patient-ventilator interaction
When PSIGNAL is displayed in real-time along with PAW and flow, the user obtains a comprehensive visual feedback about the instant state of patient-ventilator interaction (e. g., Fig. 5, top). Excessive trigger delay, cycling-off delay and ineffective efforts would be immediately obvious. Such feedback can be readily used to optimize ventilator settings. For example, in the presence of excessive cycling-off delay the flow threshold for cycling-off in PSV may be gradually increased until reasonable synchrony is observed on the screen. By optimizing cycling-off it may be expected that ineffective efforts, if any, would be reduced and trigger delay and dynamic hyperinflation improved [24]. The onset of inspiratory effort while flow is still expiratory would clearly indicate the presence of intrinsic PEEP. The time difference between onset of effort and onset of inspiratory flow may be used to optimize external PEEP and reduce dynamic hyperinflation. Brief transients in PSIGNAL that have the frequency of heart rate, along with absence of much larger transients that reflect efforts, would indicate autotriggering by cardiac artifacts resulting in overassist and apnea and would be countered by decreasing ventilator's trigger sensitivity. By freezing the screen at low speed the clinician would be able to count the actual respiratory rate of the patient.
Top Airway pressure (PAW), flow, monitor-generated signal (PSIGNAL) and diaphragm pressure (PDI) in a patient while on pressure support (PSV) and later on proportional assist (PAV). Note the marked discrepancy between ventilator rate and patient's rate during PSV. Note also that efforts were very weak on PSV, indicating a high level of assist; Bottom Plot of different rates against time spanning the transition from PSV to PAV. Note that monitor's estimate of patient's rate (RRMONITOR) accurately tracks respiratory rate determined from PDI (RRPDI). In this case the monitor clearly indicates that the increase in ventilator rate (RRVENT) following the transition was not related to an increase in patient's rate (in fact patient's rate decreased). All values are 1-min averages of rate. This accounts for the fact that RRVENT appeared to increase gradually (over 1 min) following transition. In fact the change in ventilator rate was instantaneous, before there was any increase in PDI. Event marks in PSIGNAL are real-time TONSET instances (upward marks) and TENDs (downward marks) as identified by the monitor
Such visual feedback may be supplemented by numerical displays of the relevant variables. The current study shows that retrospective TONSET and TEND are fairly close to the actual onset and end of effort identified from PDI/PMUS (Table 1). These retrospective values can therefore be used for automatic measurement of triggering and cycling-off delays. The study has also shown that the respiratory rate counted by the monitor faithfully reflects patient's respiratory rate (Fig. 4). When these values are stored, trends over time can be obtained. These may help troubleshoot respiratory distress. Furthermore, it would be possible to determine whether an increase in ventilator rate that occurs in the course of weaning trials or following a change in ventilator settings (e. g., a change in cycling-off threshold in PSV or a switch to PAV) is due simply to improved synchrony and hence may be ignored, or reflects a true change in patient's respiratory rate (i. e., distress). Since a large increase in ventilator rate during weaning trials is usually interpreted as weaning failure, such information may reduce instances of false weaning failure. Figure 5 shows a striking example of a large change in ventilator rate following a mode switch that was identified by the monitor as due to improved synchrony.
The current approach was not intended or designed to provide accurate estimates of respiratory mechanics or of the amplitude of PMUS. It was of interest, however, to see the extent to which KF and KV agreed with actual resistance and elastance. The correlations were highly significant (p < 0.0001), but there was considerable scatter (Fig. S5, ESM). There are several possible reasons for the observed differences, and some of these are correctable. It remains to be seen whether the agreement between the improvised and actual values can be improved enough to make KV and KF useful surrogates for elastance and resistance. Because errors in estimated elastance and resistance result in errors in estimated PMUS amplitude, until such improvement is achieved, the actual amplitude of PSIGNAL should not be used to reflect actual PMUS.
In summary, this study shows that a signal generated by applying the equation of motion, using improvised values of elastance and resistance, makes it possible non-invasively to identify the onsets and ends of inspiratory efforts with reasonable accuracy. Such signal may be used to synchronize the ventilator with the patient or to simply provide information on patient-ventilator interaction that may be helpful in making appropriate ventilator adjustments and in evaluating the patient, such as by providing actual patient's respiratory rate.
References
Leung P, Jubran A, Tobin MJ (1997) Comparison of assisted ventilator modes on triggering, patient effort, and dyspnea. Am J Respir Crit Care Med 155:1940–1948
Tobin MJ, Jubran A, Laghi F (2001) Patient-ventilator interaction. Am J Respir Crit Care Med 163:1059–1063
Kondili E, Prinianakis G, Georgopoulos D (2003) Patient-ventilator interaction. Br J Anaesthesia 91:106–119
Prinianakis G, Kondili E, Georgopoulos D (2003) Effects of the flow waveform method of triggering and cycling on patient-ventilator interaction during pressure support. Intensive Care Med 29:1950–1959
Giannouli E, Webster K, Roberts D, Younes M (1999) Response of ventilator-dependent patients to different levels of pressure support and proportional assist. Am J Respir Crit Care Med 159:1716–1725
Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L (2006) Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med 32:1515–1522
Younes M (2006) Proportional assist ventilation. In: Tobin MJ (ed) Principles and practice of mechanical ventilation, 2nd edn. McGraw-Hill, New York, pp 335–364
Younes M (2003) Method and device for monitoring and improving patient-ventilator interaction. PCT application #PCT/CA 2003/000976
Mead J, Agostoni E (1964) Dynamics of breathing. In: Fenn WO, Rahn H (eds) Handbook of physiology: respiration. American Physiological Society. Washington, pp 411–427
Younes M (1992) Proportional assist ventilation, a new approach to ventilatory support. Theory. Am Rev Respir Dis 145:114–120
Grasso S, Ranieri VM, Brochard L, Richard GC, Mancebo J, Slutsky AS, Younes M (2001) Closed loop proportional assist ventilation (PAV): results of a phase II multicenter trial. Am J Respir Crit Care Med 163:303A
Campbell EJM (1958) The respiratory muscles and mechanics of breathing. Loyd-Luke, London
Appendini L, Patessio A, Zanaboni S, Carone M, Gukov B, Donner CF, Rossi A (1994) Physiologic effects of positive end-expiratory pressure and mask pressure support during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 149:1069–1076
Younes M (1993) Patient-ventilator interaction with pressure-assisted modalities of ventilatory support. Semin Respir Med 14:299–322
Mancebo J (2003) Triggering and cycling off during pressure support ventilation: simplicity or sophistication? Intensive Care Med 29:1871–1872
Imanaka H, Nishimura M, Takeuchi M, Kimball WR, Yahagi N, Kumon K (2000) Autotriggering caused by cardiogenic oscillation during flow-triggered mechanical ventilation. Crit Care Med 28:402–407
Younes M, Remmers JE (1981) Control of tidal volume and respiratory frequency. In: Hornbein T (ed) Control of breathing. Lung biology in health and disease, vol. 17 Dekker, New York. pp 617–667
Agostoni E, Citterio G, D'Angelo E (1979) Decay rate of inspiratory muscle pressure during expiration in man. Respir Physiol 36:269–285
Younes M. Kun J, Masiowski B, Webster K, Roberts D (2001) A method for noninvasive determination of inspiratory resistance during proportional assist ventilation. Am J Respir Crit Care Med 163:829–839
Younes M, Webster K, Kun J, Roberts D, Masiowski B (2001) A method for measuring passive elastance during proportional assist ventilation. Am J Respir Crit Care Med 164:50–60
Fernandez F, Mendez M, Younes M (1999) Effect of ventilator flow-rate on respiratory timing in normal subjects. Am J Respir Crit Care Med 159:710–719
Nava S, Bruschi C, Rubini F, Palo A, Iotti G, Braschi A (1995) Respiratory response to inspiratory effort during pressure support ventilation in COPD patients. Intensive Care Med 21:871–879
Yamada Y, Shigeta M, Suwa K, Hanaoka K (1994) Respiratory muscle pressure analysis in pressure-support ventilation. J Appl Physiol 77:2237–2243
Younes M, Kun J, Webster K, Roberts D (2002) Response of ventilator dependent patients to delayed opening of exhalation valve. Am J Respir Crit Care Med 166:21–30
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by YRT Limited.
Electronic supplementary material
ESM 1
(DOCX 369 kb)
Rights and permissions
About this article
Cite this article
Younes, M., Brochard, L., Grasso, S. et al. A method for monitoring and improving patient: ventilator interaction. Intensive Care Med 33, 1337–1346 (2007). https://doi.org/10.1007/s00134-007-0681-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0681-4