Abstract
Objective
To test the hypothesis that levosimendan increases systemic and intestinal oxygen delivery (DO2) and prevents intramucosal acidosis in septic shock.
Design
Prospective, controlled experimental study.
Setting
University-based research laboratory.
Subjects
Nineteen anesthetized, mechanically ventilated sheep.
Interventions
Endotoxin-treated sheep were randomly assigned to three groups: control (n = 7), dobutamine (10 μg/kg/min, n = 6) and levosimendan (100 μg/kg over 10 min followed by 100 μg/kg/h, n = 6) and treated for 120 min.
Measurements and main results
After endotoxin administration, systemic and intestinal DO2 decreased (24.6 ± 5.2 vs 15.3 ± 3.4 ml/kg/min and 105.0 ± 28.1 vs 55.8 ± 25.9 ml/kg/min, respectively; p < 0.05 for both). Arterial lactate and the intramucosal–arterial PCO2 difference (ΔPCO2) increased (1.4 ± 0.3 vs 3.1 ± 1.5 mmHg and 9 ± 6 vs 23 ± 6 mmHg mmol/l, respectively; p < 0.05). Systemic DO2 was preserved in the dobutamine-treated group (22.3 ± 4.7 vs 26.8 ± 7.0 ml/min/kg, p = NS) but intestinal DO2 decreased (98.9 ± 0.2 vs 68.0 ± 22.9 ml/min/kg, p < 0.05) and ΔPCO2 increased (12 ± 5 vs 25 ± 11 mmHg, p < 0.05). The administration of levosimendan prevented declines in systemic and intestinal DO2 (25.1 ± 3.0 vs 24.0 ± 6.3 ml/min/kg and 111.1 ± 18.0 vs 98.2 ± 23.1 ml/min/kg, p = NS for both) or increases in ΔPCO2 (7 ± 7 vs 10 ± 8, p = NS). Arterial lactate increased in both the dobutamine and levosimendan groups (1.6 ± 0.3 vs 2.5 ± 0.7 and 1.4 ± 0.4 vs. 2.9 ± 1.1 mmol/l, p = NS between groups).
Conclusions
Compared with dobutamine, levosimendan increased intestinal blood flow and diminished intramucosal acidosis in this experimental model of sepsis.
Similar content being viewed by others
Introduction
Hemodynamic alterations that occur in sepsis and septic shock are complex, affecting both the heart and the peripheral circulation [1]. Although fluid resuscitation is the cornerstone of hemodynamic management of septic shock, tissue hypoperfusion can occur even after vigorous volume replacement [2], and the infusion of vasoactive drugs is usually required. Covert tissue dysoxia may persist even after hemodynamic variables have been normalized. For example, adrenergic agents might stabilize arterial blood pressure and cardiac output but can impair gut perfusion, and so fail to correct intramucosal acidosis [3, 4]. A new therapeutic strategy for the treatment of septic shock that avoids tissue hypoperfusion has been proposed recently, viz. the recruitment of the microcirculation with vasodilators [5].
Levosimendan, a new calcium-sensitizing inotropic drug, has been shown to improve cardiac function and survival in patients with congestive heart failure [6]. Levosimendan also acts as a vasodilator by stimulating (ATP)-sensitive potassium channels in vascular smooth muscle cells [7]. A preliminary report in hypodynamic experimental endotoxemic shock showed that levosimendan improved systemic and intestinal oxygen transport [8]. In addition, recent clinical research has shown that levosimendan improves systemic hemodynamics and regional perfusion in septic patients with cardiac dysfunction unresponsive to dobutamine [9].
We previously reported that high doses of levosimendan improve oxygen transport and prevent the development of intramucosal acidosis in a normodynamic sheep model of endotoxemia. We also noted that systemic hypotension and lactic acidosis developed in these animals, a phenomenon attributed to levosimendan-induced excessive vasodilation [10].
Our present hypothesis is that levosimendan, at doses lower than those used in our previous study, has a salutary effect in sepsis by avoiding elevations in intramucosal-arterial PCO2 difference (ΔPCO2) through increases in systemic and intestinal oxygen transport without producing hypotension or elevations in lactate. To test this hypothesis, we compared the oxygen transport and hemodynamic responses of an experimental model of septic shock infused with levosimendan or dobutamine. We chose the latter because this inotrope is commonly used to increase tissue perfusion in septic shock. This work has been previously presented in abstract form [11].
Materials and methods
Surgical preparation
Each of 19 sheep (21 ± 3 kg) were anesthetized with 30 mg/kg of sodium pentobarbital, intubated and mechanically ventilated (Dual Phase Control Respirator Pump Ventilator, Harvard Apparatus, South Natick, Mass, USA) with a tidal volume of 15 ml/kg, a FIO2 of 0.21 and a PEEP of 8 cmH2O. The respiratory rate was set to keep the end-tidal PCO2 at 35 mmHg. Neuromuscular blockade was performed with intravenous pancuronium bromide (0.06 mg/kg). Additional pentobarbital boluses (1 mg/kg/h) were administered as required.
Catheters were advanced through the left femoral vein to administer fluids and drugs, and through the left femoral artery to measure blood pressure and to obtain blood gases. A pulmonary artery catheter was inserted through right external jugular vein (Flow-directed thermodilution fiberoptic pulmonary artery catheter, Abbott Critical Care Systems, Mountain View, CA, USA).
An orogastric tube was inserted to allow drainage of gastric contents, followed by a midline laparotomy and splenectomy. An electromagnetic flow probe was placed around the superior mesenteric artery to measure intestinal blood flow. A catheter was placed in the mesenteric vein through a small vein proximal to the gut to draw blood gases. A tonometer was inserted through a small ileotomy to measure intramucosal PCO2, and the abdominal wall incision was closed after careful hemostasis.
Measurements and derived calculations
Arterial, systemic, pulmonary and central venous pressures were measured with corresponding transducers (Statham P23 AA, Statham, Hato Rey, Puerto Rico). Cardiac output (Q) was measured by thermodilution with 5 ml of 0 °C saline solution (HP OmniCare Model 24 A 10, Hewlett Packard, Andover, MA, USA). The average of three measurements taken randomly during the respiratory cycle was normalized to body weight. Intestinal blood flow was measured by the electromagnetic method (Spectramed Blood Flowmeter model SP 2202 B, Spectramed, Oxnard, CA, USA) with in-vitro calibrated transducers of 5–7 mm of diameter (Blood Flowmeter Transducer, Spectramed, Oxnard, CA, USA). Occlusive zero was controlled before and after each experiment. Non-occlusive zero was corrected before each measurement. Superior mesenteric blood flow was normalized to gut weight (Qintestinal).
Arterial, mixed venous and mesenteric venous PO2, PCO2 and pH were measured with a blood gas analyzer (ABL 5, Radiometer, Copenhagen, Denmark), and hemoglobin and oxygen saturation were measured with a cooximeter calibrated for sheep blood (OSM 3, Radiometer, Copenhagen, Denmark). Arterial, mixed venous and mesenteric venous contents (CaO2, CvO2 and CvmO2, respectively) were calculated as: Hb × 1.34 × O2 saturation + PO2 × 0.0031. Systemic and intestinal DO2 and VO2 (DO2, VO2, DO2i and VO2i, respectively) were calculated as DO2 = Q × CaO2; VO2 = Q × (CaO2 – CvO2); DO2i = Qintestinal × CaO2; and VO2i = Qintestinal × (CaO2 – CvmO2).
Intramucosal PCO2 was measured with a tonometer (Tonometrics® Catheter, Datex Ohmeda Division, Helsinki, Finland) filled with 2.5 ml of saline solution. Of this quantity, 1.0 ml was discarded after an equilibration period of 30 min, and PCO2 was measured in the remaining 1.5 ml. Its value was corrected to the corresponding equilibration period and was used to calculate ΔPCO2.
Arterial lactate, sodium, potassium, chloride and serum total proteins were measured with an automatic analyzer (Automatic Analyzer Hitachi 912, Boehringer Mannheim Corporation, Indianapolis, IN, USA). Anion gap was calculated as ([Na+] + [K+]) – ([Cl–] + [HCO3 –]). Anion gap was corrected according changes in plasma protein concentration [12].
Experimental procedure
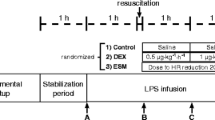
Basal measurements were taken after a stabilization period of no less than 30 min. Then, FIO2 was increased to 0.50 and 5 μg/kg of E. coli lipopolysaccharide (Sigma Chemical Co., St. Louis, MO, USA) was infused in 10 min followed by an infusion of 2 μg/kg/h for 2 h. The endotoxic sheep were assigned randomly to three groups: (1) Control, n = 7; (2) dobutamine (Eli Lilly, Indianapolis, IN, USA), n = 6 (10 μg/kg/min); (3) levosimendan (Orion Pharma, Espoo, Finland), n = 6 (loading dose of 100 μg/kg in 10 min, followed by continuous infusion of 100 μg/kg/h throughout the rest of the experiment). Drug infusions were started immediately after the loading dose of endotoxin. Dobutamine infusion of 10 μg/kg/min was chosen because this rate is commonly used, both in clinical and experimental settings, to increase cardiac output. The dose of levosimendan used was half that of our prior study [10]. All groups were infused with the same volume of saline solution (20 ml/kg/h).
Measurements of hemodynamics, oxygen transport and ΔPCO2 were performed at 30-min intervals during a period of 120 min from the start of endotoxin administration. Determinations of lactate, sodium, potassium, chloride and serum total proteins were performed hourly.
At the end of the experiment, the animals were killed with an additional dose of pentobarbital and a potassium chloride bolus. A catheter was inserted in the superior mesenteric artery and Indian ink was instilled through it. Dyed intestinal segments were dissected, washed and weighed to calculate gut indexes.
Care of animals was in accordance with National Institute of Health guidelines.
Statistical analysis
Data were assessed for normality and expressed as mean ± standard deviation (SD). Differences within groups were analyzed using repeated measures of ANOVA and Dunnett's multiple comparisons test to compare each time point to basal. One-time comparisons between groups were tested using one-way ANOVA and Newman–Keuls multiple comparison test. The software GraphPad PRISM version 3.02 was used.
Results
Hemodynamic effects
All animals survived the experiments. As shown in Table 1 and Fig. 1, in the control group, cardiac output and intestinal blood flow decreased without changes in systemic vascular resistance. Pulmonary vascular resistance increased. Dobutamine maintained cardiac output but intestinal blood flow decreased as in the control group. Levosimendan avoided decreases in both cardiac output and intestinal blood flow. Stroke volume was similar in all groups. Dobutamine and levosimendan both increased heart rate, but this effect was more pronounced in the dobutamine group. Both agents diminished systemic and pulmonary vascular resistances. The fraction of cardiac output directed to the intestine was lower in the dobutamine group than in the control and levosimendan groups.
Effects on oxygen transport
As shown in Fig. 2, endotoxin reduced systemic and intestinal DO2. Dobutamine preserved systemic DO2 but intestinal DO2 fell; levosimendan maintained systemic and intestinal DO2. Changes from baseline mixed venous and mesenteric venous oxygen saturation following changes in systemic and intestinal DO2 are shown in Fig. S1 of the electronic supplementary material (ESM).
Behavior of systemic and intestinal oxygen parameters in basal conditions, and after endotoxin administration in control, dobutamine and levosimendan groups. a Systemic oxygen transport; b intestinal oxygen transport; c systemic oxygen consumption; d intestinal oxygen consumption. Data are shown as mean ± SD. * p < 0.05 vs basal, § p < 0.05 vs control, # p < 0.05 vs control and dobutamine
Effects on ΔPCO2
As shown in Fig. 3, ΔPCO2 increased in control and dobutamine groups. Levosimendan precluded the elevation of ΔPCO2. Changes from baseline are shown in Fig. S2 of the ESM.
Metabolic effects
As shown in Table 2, a similar degree of metabolic acidosis developed in all groups. This was explained by equivalent proportions of hyperchloremia (increased [Cl–]/[Na+] relationship) and increased anion gap. Hyperlactatemia appeared in the three groups. Despite the lack of statistical significance, hyperlactatemia was somewhat higher in control and levosimendan groups. Changes in anion gap were only partially explained by the elevations in lactate. Changes from baseline arterial base excess, anion gap, lactate and [Cl–]/[Na+] relationship are shown in Fig. S3.
Effects on pulmonary oxygenation
As shown in Table 2 and Fig. 4, endotoxin induced a severe derangement in pulmonary oxygenation, which was prevented by either dobutamine or levosimendan. Pulmonary and extrapulmonary determinants of arterial PO2 were more severely compromised in the control group than in the dobutamine and levosimendan groups. At 120 min, venous admixtures were 25 ± 21, 7 ± 3 and 9 ± 6%, respectively (p < 0.05) and mixed venous PO2 32 ± 5, 51 ± 11 and 47 ± 8 mmHg, in control, dobutamine and levosimendan groups respectively (p < 0.05).
Discussion
The main finding of this study is that both levosimendan and dobutamine preserved cardiac output and systemic oxygen transport in this model of septic shock. However, levosimendan alone was able to prevent the reduction in intestinal blood flow and diminished the development of intramucosal acidosis. On the other hand, dobutamine decreased the fraction of cardiac output directed to the gut.
Hemodynamic effects
As previously described [13], the more evident effect of endotoxin in the control group was the development of a low-flow state with a marked increase in pulmonary vascular resistance. Systemic vascular resistance did not increase despite decreased cardiac output, perhaps because of the relaxing endotoxin effect on vascular tone [13].
As there were no changes in stroke volume, the beneficial effects of levosimendan and dobutamine on cardiac output may be related to an induced tachycardia, a response that may not be optimal from a myocardial energy metabolism point of view. Preservation of cardiac output, with reduction in systemic and pulmonary pressures and resistances, imply that both drugs behaved as systemic and pulmonary vasodilators.
The effects of levosimendan on cardiac output are usually ascribed to enhanced contractility, and, additionally, to systemic and pulmonary vasodilation, although stroke volume did not improve in our experiments. In a previous study, levosimendan could not restore depressed cardiac output to basal levels after endotoxin administration, yet cardiac output remained at higher levels than in endotoxemic controls [8]. In a study in endotoxin-exposed guinea pigs, levosimendan failed to reverse left ventricular dysfunction [14]. In earlier experiments using a higher dose, levosimendan also failed to increase stroke volume [10]. A possible explanation for these effects is that acidosis may have blunted the inotropic effect of levosimendan [15]. In addition, a study in normal dogs showed that levosimendan increased cardiac output by tachycardia, without changes in stroke volume [16].
Dobutamine is the recommended drug for septic patients whose cardiac output remains low despite adequate fluid resuscitation [17]. In our experiments, its effects on cardiac output were similar to those of levosimendan. On the other hand, dobutamine infusion resulted in greater tachycardia, greater arterial hypotension and more blood flow redistribution from the gut than were found with levosimendan. Although dobutamine was thought to be a relatively selective β1 receptor agonist, it is now clear that its pharmacological effects are complex. In the formulation available for clinical use, dobutamine is a racemic mixture of a (–) isomer that acts as a α1 receptor agonist, able to cause marked pressor responses, and a (+) isomer that behaves as a α1 antagonist that can block the preceding effects. In animals, rates of administration of 2.5–15 μg/kg/min increase cardiac output with minor changes in systemic vascular resistance [18]. In septic patients, dobutamine might cause arterial hypotension. In a controlled study, the use of dobutamine to increase oxygen transport was associated with higher requirements for noradrenaline (1.2 vs 0.23 μg/kg/min) [19], so dobutamine might behave as a systemic vasodilator in sepsis. In addition, its effects on pulmonary vasculature are controversial [20].
As a consequence of levosimendan- and dobutamine-induced vasodilation, relative hypovolemia might be present in this experimental design. However, central venous and pulmonary wedge pressures did not decrease in either group. Notwithstanding this, a more aggressive fluid resuscitation could have induced different hemodynamic effects.
Effects on oxygen transport and tissue perfusion
Parallel to changes in cardiac output and intestinal blood flow, endotoxin decreased systemic and intestinal DO2. Although systemic and intestinal VO2 remained unchanged, there was evidence of tissue dysoxia and hypoperfusion, evidenced by the development of metabolic acidosis of a comparable magnitude in all groups. Approximately half of base excess reduction might be attributed to hyperchloremia probably caused by saline administration [21], as implied by the increased [Cl–]/[Na+] relationship [22]. The elevation in the anion gap accounted for the remaining component. Increased aerobic glycolysis [23], pulmonary production of lactate [24], increase of unmeasured anions of unknown source [25], or disturbed energetic metabolism in sepsis, so-called cytopathic hypoxia [26], are all possible underlying mechanisms. The failure of dobutamine- and levosimendan-induced increases in cardiac output to prevent metabolic acidosis and hyperlactatemia supports the conclusion that these metabolic manifestations of dysoxia are not solely related to systemic hypoperfusion but rather to some of the mechanisms previously discussed.
Another important consequence of endotoxemia was intramucosal acidosis. The mechanisms responsible for increases in ΔPCO2 in sepsis are controversial. In some experimental models, intramucosal acidosis reflects low blood flow and tissue dysoxia [27, 28]. On the other hand, VanderMeer et al. found that intramucosal acidosis developed despite preservation of blood flow and tissue PO2 in endotoxemic pigs, a phenomenon attributed to cytopathic hypoxia [29]. Vallet et al. [30] and Dubin et al. [31, 32] showed that hypoperfusion is a key factor in the development of venous and tissue hypercarbia, and, in this way, an increase in blood flow was shown to prevent intramucosal acidosis in sheep endotoxemia [33]. In addition, Tugtekin et al. showed an association between increased ΔPCO2 and diminished villi microcirculation in endotoxemic pigs [34]. The findings of this study reinforces that ΔPCO2 is mainly dependent on perfusion. On the other hand, hyperlactatemia seems to be a metabolic expression unresponsive to increased blood flow.
By increasing systemic blood flow, and/or by a direct, local vasodilatory effect, levosimendan may reduce the increase in ΔPCO2. This effect on regional perfusion has been reported in septic patients with myocardial depression unresponsive to dobutamine [9], and in endotoxemic sheep [10]. Schwarte et al. have recently reported that levosimendan increased microvascular gastric mucosal oxygenation in normal dogs, without significant changes in oxygen transport, and that dobutamine produced similar effects only after striking elevations of cardiac output [16]. However, in another study, levosimendan could not correct intramucosal acidosis despite increased gut blood flow [8].
In the present study, dobutamine had no effect on ΔPCO2, in agreement with its actions on intestinal blood flow. These findings contradict clinical and experimental studies showing beneficial effect of dobutamine on intestinal perfusion and intramucosal acidosis [4, 35, 36]. Conversely, other investigators have shown that dobutamine might decrease mesenteric perfusion. Heino et al. demonstrated that dobutamine worsens splanchnic tissue perfusion during partial superior mesenteric artery occlusion [37]. Hiltebrand et al. showed that dobutamine fails to increase intestinal blood flow in pigs with fecal peritonitis [38]. In addition, clinical studies by Lebuffe et al. [39] and Morelli et al. [9] showed that dobutamine, in septic patients, does not improve ΔPCO2 significantly. Discrepancies between studies might be due to different clinical or experimental situations, or to the presence of hypovolemia. Because hypovolemia has a strong vasoconstricting stimulus, especially for the splanchnic bed, it could add to the α-adrenergic effects of dobutamine in subjects that have not been adequately resuscitated with fluids [37]. The slightly lower baseline cardiac output and intestinal blood flow suggest that dobutamine-treated sheep could be hypovolemic. Indeed, a more aggressive resuscitation regimen with fluids might have produced different results. Neviere et al. [40] and De Backer et al. [41] have also studied the effects of dobutamine in endotoxemic shock but, differently to our experiments, the animals were more aggressively resuscitated. In both studies, dobutamine increased mesenteric blood flow compared with saline alone. Nevertheless, despite the improvement in intramucosal acidosis, dobutamine failed to normalize intramucosal pH, which remained considerably lower than basal values. Different effects of dobutamine and levosimendan on intestinal perfusion and ΔPCO2 can be ascribed to their effects on flow redistribution.
Effects on pulmonary oxygenation
As expected, endotoxin caused lung injury with severe compromise of gas exchange and pulmonary hypertension [13]. This lesion is mediated by inflammatory and hydrostatic factors [42]. Levosimendan and dobutamine might have increased venous admixture because of their hemodynamic effects [43], but in fact it was reduced, probably due to lowered capillary pulmonary pressure and edema formation. As mixed venous PO2 increased, the final result was a rise in arterial PO2. In addition, dobutamine might have induced a decrease in pulmonary edema because of its beta-adrenergic stimulation of alveolar epithelial sodium and fluid transport [44]. Levosimendan, however, increased arterial PCO2, as a probable consequence of increased deadspace fraction, which is a well-known risk factor for death in acute respiratory distress syndrome [45].
Limitations of this study
This study has some important limitations. The model was a short-term endotoxin infusion that resulted in a hypodynamic state. Long-term endotoxin infusion may produce a different hemodynamic profile that more adequately resembles human septic shock [34]. In addition, the administration of levosimendan and dobutamine for a longer period of time after endotoxin might have produced different results. The administration of drugs just after endotoxin administration does not represent medical practice. Consequently, neither the model nor the timing of drug administration adequately approach human septic shock. In addition, the lack of intraabdominal pressure measurements might be another limitation of this study. Intraabdominal hypertension is likely in this model and an imbalance between groups might affect intestinal blood flow and its response to drugs [46]. Finally, small sample size might mask some differences between the groups.
Conclusions
In this experimental model of sheep endotoxemia, levosimendan prevented the decreases of systemic and intestinal oxygen transport and diminished the development of intramucosal acidosis. In addition, it corrected pulmonary hypertension. These results justify further clinical trials to assess the use of levosimendan in septic shock.
References
Parrillo JE (1993) Pathogenetic mechanisms of septic shock. N Engl J Med 328:1471–1477
Fiddian-Green RG, Haglund U, Gutierrez G, Shoemaker WC (1993) Goals for the resuscitation of shock. Crit Care Med 21:S25–S31
Mark P, Mohedin M (1994) The contrasting effects of dopamine and norepinephrine on systemic and splanchnic oxygen utilization in hyperdynamic sepsis. JAMA 272:1354–1357
Neviere R, Mathieu D, Chagnon JL, Lebleu N, Wattel F (1996) The contrasting effects of dobutamine and dopamine on gastric mucosal perfusion in septic patients. Am J Respir Crit Care Med 154:1684–1688
Buwalda M, Ince C (2002) Opening the microcirculation: Can vasodilators be useful in sepsis? Intensive Care Med 28:1208–1217
Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP, Lehtonen L, Steering Committee and Investigators of the Levosimendan Infusion versus Dobutamine (LIDO) Study (2002) Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 360:196–202
Yokoshiki H, Katsube Y, Sunagawa M, Sperelakis N (1997) Levosimendan, a novel Ca2 + sensitizer, activates the glibenclamide-sensitive K+ channel in rat arterial myocytes. Eur J Pharmacol 333:249–259
Oldner A, Konrad D, Weitzberg E, Rudehill A, Rossi P, Wanecek M (2001) Effects of levosimendan, a novel inotropic calcium-sensitizing drug, in experimental septic shock. Crit Care Med 29:2185–2193
Morelli A, De Castro S, Teboul JL, Singer M, Rocco M, Conti G, De Luca L, Di Angelantonio E, Orecchioni A, Pandian NG, Pietropaoli P (2005) Effects of levosimendan on systemic and regional hemodynamics in septic myocardial depression. Intensive Care Med 31:638–644
Dubin A, Maskin B, Murias G, Pozo MO, Sottile JP, Baran M, Edul VS, Canales HS, Estenssoro E (2006) Effects of levosimendan in normodynamic endotoxemia: a controlled experimental study. Resuscitation 69:277–286
Dubin A, Murias G, Silva C, Maskin B, Sottile JP, Barán M, Pozo M, Kanoore Edul VS, Canales HS, Etcheverry G, Estenssoro E (2004) Effects of levosimendan in endotoxemic sheep: a controlled study. Intensive Care Medicine 30:S103
Constable PD (2001) Total weak acid concentration and effective dissociation constant of nonvolatile buffers in human plasma. J Appl Physiol 91:1364–1371
Traber DL, Flynn JT, Herndon DN, Redl H, Schlag G, Traber LD (1989) Comparison of cardiopulmonary responses to single bolus and continuous infusion of endotoxin in an ovine model. Circ Shock 27:123–138
Behrends M, Peters J (2003) The calcium sensitizer levosimendan attenuates endotoxin-evoked myocardial dysfunction in isolated guinea pig hearts. Intensive Care Med 29:1802–1807
Takahashi R, Endoh M (2002) Effects of OR-1896, a metabolite of levosimendan, on force of contraction and Ca2 + transients under acidotic condition in aequorin-loaded canine ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol 366:440–448
Schwarte LA, Picker O, Bornstein SR, Fournell A, Scheeren TWL (2005) Levosimendan is superior to milrinone and dobutamine in selectively increasing microvascular gastric mucosal oxygenation in dogs. Crit Care Med 33:135–142
Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM (2004) Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med 30:536–555
Ruffolo RR Jr (1987) Review: The pharmacology of dobutamine. Am J Med Sci 294:244–248
Hayes MA, Timmins AC, Yau E, Palazzo M, Hinds CJ, Watson D (1994) Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med 330:1717–1722
Pagnamenta A, Fesler P, Vandinivit A, Brimioulle S, Naeije R (2003) Pulmonary vascular effects of dobutamine in experimental pulmonary hypertension. Crit Care Med 31:1140–1146
Kellum JA (2002) Fluid resuscitation and hyperchloremic acidosis in experimental sepsis: improved short-term survival and acid-base balance with Hextend compared with saline. Crit Care Med 30:300–305
Durward A, Skellett S, Mayer A, Taylor D, Tibby SM, Murdoch IA (2001) The value of the chloride:sodium ratio in differentiating the aetiology of metabolic acidosis. Intensive Care Med 27:828–835
Levy B, Gibot S, Franck P, Cravoisy A, Bollaert PE (2005) Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet 365:871–875
Brown SD, Clark C, Gutierrez G (1996) Pulmonary lactate release in patients with sepsis and the adult respiratory distress syndrome. J Crit Care 11:2–8
Rackow EC, Mecher C, Astiz ME, Goldstein C, McKee D, Weil MH (1990) Unmeasured anion during severe sepsis with metabolic acidosis. Circ Shock 30:107–115
Fink M (2001) Cytopathic hypoxia. Mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Crit Care Clin 17:219–237
Antonsson JB, Engstrom L, Rasmussen I, Wollert S, Haglund UH (1995) Changes in gut intramucosal pH and gut oxygen extraction ratio in a porcine model of peritonitis and hemorrhage. Crit Care Med 23:1872–1881
Vallet B, Lund N, Curtis SE, Kelly D, Cain SM (1994) Gut and muscle tissue PO2 in endotoxemic dogs during shock and resuscitation. J Appl Physiol 76:793–800
VanderMeer TJ, Wang H, Fink MP (1995) Endotoxemia causes ileal mucosal acidosis in the absence of mucosal hypoxia in a normodynamic porcine model of septic shock. Crit Care Med 23:1217–1226
Vallet B, Teboul JL, Cain S, Curtis S (2000) Venoarterial CO2 difference during regional ischemic or hypoxic hypoxia. J Appl Physiol 89:1317–1321
Dubin A, Murias G, Estenssoro E, Canales H, Badie J, Pozo M, Sottile JP, Baran M, Palizas F, Laporte M (2002) Intramucosal-arterial PCO2 gap fails to increase during hypoxic hypoxia. Critical Care 6:514–520
Dubin A, Estenssoro E, Murias G, Pozo MO, Sottile JP, Baran M, Piacentini E, Canales HS, Etcheverry G (2004) Intramucosal-arterial PCO2 gradient does not reflect intestinal dysoxia in anemic hypoxia. J Trauma 57:1211–1217
Dubin A, Murias G, Maskin B, Pozo MO, Sottile JP, Baran M, Edul VS, Canales HS, Badie JC, Etcheverry G, Estenssoro E (2005) Increased blood flow prevents intramucosal acidosis in sheep endotoxemia: A controlled study. Critical Care 9:R66–R73
Tugtekin IF, Radermacher P, Theisen M, Matejovic M, Stehr A, Ploner F, Matura K, Ince C, Georgieff M, Trager K (2001) Increased ileal-mucosal-arterial PCO2 gap is associated with impaired villus microcirculation in endotoxic pigs. Intensive Care Med 27:757–766
Silva E, DeBacker D, Créteur J, Vincent JL (1998) Effects of vasoactive drugs on gastric intramucosal pH. Crit Care Med 26:1749–1758
Lisbon A (2003) Dopexamine, dobutamina, and dopamine increase splanchnic blood flow. What is the evidence? Chest 123:430S–463S
Heino A, Hartikainen J, Merasto ME, Koski EM, Tenhunen J, Alhava E, Takala J (2000) Effects of dobutamina on splanchnic tissue perfusion during partial superior mesenteric artery occlusion. Crit Care Med 28:3484–3490
Hiltebrand LB, Krejci V, Sigurdsson GH (2004) Effects of dopamine, dobutamina, and dopexamine on microcirculatory blood flow in the gastrointestinal tract during sepsis and anesthesia. Anesthesiology 100:1188–1197
Lebuffe G, Levy B, Neviere R, Chagnon JL, Perrigault PF, Duranteau J, Edouard A, Teboul JL, Vallet B (2002) Dobutamine and gastric-to-arterial carbon dioxide gap in severe sepsis without shock. Intensive Care Med 28:265–271
Neviere R, Chagnon JL, Vallet B, Lebleu N, Marechal X, Mathieu D, Wattel F, Dupuis B (1997) Dobutamine improves gastrointestinal mucosal blood flow in a porcine model of endotoxic shock. Crit Care Med 25:1371–1377
De Backer D, Zhang H, Manikis P, Vincent JL (1996) Regional effects of dobutamine in endotoxic shock. J Surg Res 65:93–100
Demling RH, Smith M, Gunther R, Flynn JT, Gee MH (1981) Pulmonary injury and prostaglandin production during endotoxemia in conscious sheep. Am J Physiol 240:H348–H353
Lynch JP, Mhyre JG, Dantzker DR (1979) Influence of cardiac output on intrapulmonary shunt. J Appl Physiol 46:315–321
Tibayan FA, Chesnutt AN, Folkesson HG, Eandi J, Matthay MA (1997) Dobutamine increases alveolar liquid clearance in ventilated rats by beta-2 receptor stimulation. Am J Respir Crit Care Med 156:438–444
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA (2002) Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 25; 346:1281–1286
Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, Bihari D, Innes R, Cohen J, Singer P, Japiassu A, Kurtop E, De Keulenaer BL, Daelemans R, Del Turco M, Cosimini P, Ranieri M, Jacquet L, Laterre PF, Gattinoni L (2004) Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med 30:822–829
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was solely funded by the Cátedra de Farmacología, Facultad de Ciencias Médicas, Universidad Nacional de La Plata.
None of the authors have any financial interests to disclose.
This article is discussed in the editorial available at: http://dx.doi.org/10.1007/s00134-006-520-z.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Dubin, A., Murias, G., Sottile, J.P. et al. Effects of levosimendan and dobutamine in experimental acute endotoxemia: a preliminary controlled study. Intensive Care Med 33, 485–494 (2007). https://doi.org/10.1007/s00134-006-0519-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0519-5