Abstract
Objective
To evaluate the efficacy of different lung recruitment maneuvers using electric impedance tomography.
Design and setting
Experimental study in animal model of acute lung injury in an animal research laboratory.
Subjects
Fourteen pigs with saline lavage induced lung injury.
Interventions
Lung volume, regional ventilation distribution, gas exchange, and hemodynamics were monitored during three different recruitment procedures: (a) vital capacity maneuver to an inspiratory pressure of 40 cmH2O (ViCM), (b) pressure-controlled recruitment maneuver with peak pressure 40 and PEEP 20 cmH2O, both maneuvers repeated three times for 30 s (PCRM), and (c) a slow recruitment with PEEP elevation to 15 cmH2O with end inspiratory pauses for 7 s twice per minute over 15 min (SLRM).
Measurements and results
Improvement in lung volume, compliance, and gas exchange were similar in all three procedures 15 min after recruitment. Ventilation in dorsal regions of the lungs increased by 60% as a result of increased regional compliance. During PCRM compliance decreased by 50% in the ventral region. Cardiac output decreased by 63±4% during ViCM, 44±2% during PCRM, and 21±3% during SLRM.
Conclusions
In a lavage model of acute lung injury alveolar recruitment can be achieved with a slow lower pressure recruitment maneuver with less circulatory depression and negative lung mechanic side effects than with higher pressure recruitment maneuvers. With electric impedance tomography it was possible to monitor lung volume changes continuously.
Similar content being viewed by others
Introduction
Lung recruitment maneuvers (RMs) are used to open up atelectasis and counteract alveolar derecruitment due to low tidal volume (TV) ventilation. Classically, rapid high-pressure RMs applied for up to 60 s have been used [1, 2, 3, 4]. However, these RMs may be associated with serious circulatory side effects [3, 5, 6], risk of baro-/volutrauma [7], and even worsening of oxygenation [8, 9]. It is possible that lung volume (LV) expansion can be achieved with less traumatic methods, i.e., less pressure maintained over a longer period [10, 11]. Several techniques have been used to monitor global lung expansion, including methods based on dilution of tracer gases such as helium [12], nitrogen [13], and sulfur hexafluoride [14]. Computed tomography (CT) is a useful technique for assessing regional aeration and alveolar recruitment [15]. Still, these techniques are intermittent and we lack a bedside tool for dynamic monitoring of lung ventilation disturbances.
Electric impedance tomography (EIT) is a technique based on the injection of small currents and voltage measurements using electrodes on the skin surface generating a cross-sectional image representing impedance changes in a slice of the thorax [16]. A high temporal resolution makes dynamic monitoring of ventilation induced LV changes possible. The anatomical resolution is less precise than that of CT but still allows for radiation-free differentiation of regional lung ventilation disturbances [17, 18, 19].
In this study we measured changes in LV and regional distribution of ventilation using EIT to evaluate whether an RM with lower pressure performed over 15 min is as effective as three repeated 30-s high-pressure RMs performed as three sustained inflations or pressure-controlled maneuvers. The study was performed in an animal model of acute lung injury (ALI) achieved by repeated bronchoalveolar lavage.
Material and methods
The study was approved by the Committee for Ethical Review of Animal Experiments in Gothenburg, Sweden, and performed in accordance with National Institutes of Health guidelines. Fourteen pigs (25–30 kg) were included.
Anesthesia
Animals were premedicated using 15 mg/kg ketamine (Ketalar, Park-Davis, Sweden) and 0.3 mg/kg midazolam (Dormicum, Roche, Switzerland) intramuscularly. General anesthesia was induced with 6 mg/kg pentobarbital sodium (Apoteksbolaget, Sweden) followed by hourly infusions of 4 mg/kg and 25 µg/kg fentanyl (Fentanyl Pharmalink, Pharmalink, Sweden). Muscle relaxation was achieved by 0.15 mg/kg pancuronium (Pavulon, Organon, Sweden) as a bolus followed by 0.3 mg/kg per hour. The pigs were tracheotomized with an 8-mm endotracheal tube. Mechanical ventilation was performed using a Servo 300 ventilator (Siemens-Elema, Sweden), volume-controlled mode (VCV), TV 10 ml/kg, and inspiratory oxygen fraction of 0.5. Normovolemia was maintained by hourly infusion of 10 ml/kg Ringer’s solution with 2.5% glucose. Prior to recruitment the animals were volume expanded using 8 ml/kg colloid infusion (Voluven, Fresenius Kabi, Sweden). The study was performed with animals in supine position. After the protocol the animals were killed during deepened anesthesia.
Preparation
Arterial and central venous lines were surgically placed and a pulmonary artery catheter (CCOmbo/SVO2, Edwards Life Sciences, Calif., USA) inserted via the right internal jugular vein. We recorded data on heart rate, mean arterial pressure, central venous pressure, mean pulmonary artery pressure, and mixed venous oxygen saturation. Cardiac output was measured using bolus thermodilution technique. During the RMs measurements were performed as single boluses at the end of each 30-s period. Arterial oxygen (PaO2), carbon dioxide (PaCO2) pressure, and pH were monitored by a Trend CareMonitor 6000 sensor (Diametrics Medical, UK) inserted through a femoral artery catheter. Oxygen saturation was recorded using a pulsoximeter. Respiratory rate, volumes, and pressures were measured using side stream spirometry. Tracheal pressure (Ptrach) was measured at the tip of the endotracheal tube [20]. Inspiratory and expiratory fractions of oxygen and carbon dioxide were determined by paramagnetic and infrared technology (AS/3, Datex-Ohmeda, Finland). A modified technique for nitrogen washout/washin was used to measure end-expiratory LV (EELVN2) [21].
Electric impedance tomography
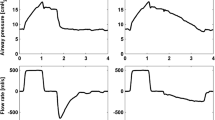
Sixteen electrocardiographic electrodes were placed around the chest wall and connected to the EIT device (Dräger/GoeMFII, Germany). EIT data were generated by injection of electrical currents of 5 mA, 50 kHz with measurements of voltage differences between neighboring electrode pairs in a sequential rotating process, where a scan was obtained every 77 ms [22]. The scan slice has an estimated thickness of 5–10 cm [16]. With a prototype software global and regional impedance changes were analyzed. In this study the electrodes were positioned at the level of the 5th intercostal space. This level was chosen in accordance with previous findings [23] where tidal amplitudes of the impedance changes were least affected by increased PEEP. Calibrations of global electrical impedance changes against known LV changes were carried out before and after lavage using a super syringe (Fig. 1).
Experimental procedure
After preparation repeated bronchoalveolar lavages with body warm saline (30 ml/kg) were performed [24, 25] with PEEP 10 cmH2O and FIO2 1.0. The procedure was continued until PaO2 was less than 26 kPa at FIO2 1.0 when PEEP was lowered to 5 cmH2O. Total amount saline ranged from 9 to 12 l. The animals were allowed to stabilize for 1 h at FIO2 0.5.
Recruitment maneuver procedures
Basal ventilation (BV) during the recruitment protocol was VCV, 10 ml/kg, RR 20/min, inspiratory to expiratory ratio (I:E) 1:2, and FIO2 0.5. Before each RM derecruitment was achieved by applying zero end-expiratory pressure, resulting in a decrease in PaO2 to below 13 kPa. RMs then started from BV with PEEP 5 cmH2O. During recovery periods within and 15 min after recruitment procedures PEEP was 10 cmH2O.
Three different RMs were performed in random order:
-
ViCM: a vital capacity maneuver in which a pressure of 40 cmH2O was applied for 30 s repeated three times with a recovery period of 30 s (BV, PEEP 10) between maneuvers
-
PCRM: a high-level pressure control maneuver in which the ventilator was set at PEEP 20 cmH2O, peak pressure 20 cmH2O above PEEP, I:E ratio 1:1, and RR 20/min was applied for 30 s and repeated three times with a recovery period of 30 s (BV, PEEP 10) between maneuvers
-
SLRM: a slow lower pressure maneuver performed for 15 min in VCV, TV 10 ml/kg, I:E 1:2, RR 20/min, PEEP 15 cmH2O and prolonged end-inspiratory pauses for 7 s twice per minute
Hemodynamic, respiratory, and gas exchange parameters as well as impedance changes were recorded continuously from baseline before each recruitment procedure, during the maneuvers, and continuing throughout the recovery period until 15 min after recruitment. EELVN2 was measured before and after 15-min recovery from the RMs, at PEEP 10. Cardiac output was measured at baseline, during recruitment, and during recovery.
Calculations and statistics
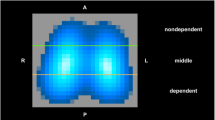
Respiratory, hemodynamic, and shunt calculations were made using standard formulae. Total respiratory compliance was calculated as the TV divided by the difference between end-inspiratory and end-expiratory tracheal pressure (ΔPtrach). From the EIT image four regions of interest (ROI)—ventral, midventral, middorsal, and dorsal—were chosen. The regional TV values (TVROI) were calculated as: TVROI=(ΔZROI/ΔZGLOB) x TV, where ΔZROI is the regional impedance change for a ROI and ΔZGLOB is the sum of the impedance changes in all ROIs (=global impedance changes). Regional compliance was obtained by dividing TVROI by ΔPtrach, assuming no flow at end inspiration and expiration.
Values are presented as mean ±SEM. Analysis of variance for repeated measures was performed, followed by Fisher’s protected least significant difference test. The paired t test was used to evaluate changes between measuring points and differences between maneuvers. Bonferroni’s correction for multiple comparisons was performed. A p value less than 0.05 was considered statistically significant.
Results
Saline lavage resulted in severe lung injury in all animals with significant decrease in PaO2 and compliance while shunt, alveolar dead space, PaCO2, mean pulmonary artery pressure, and cardiac output increased (Fig. 2). EELVN2 prior to lavage was 640±64 ml and decreased following lavage to 330±37 ml (p<0.01). Changes in global impedance were well correlated with LV changes both before and after lavage (R2>0.95).
Hemodynamic and respiratory data from 14 pigs (mean ±SEM) before (BL 0 ) and after lavage (BL LAV ; black bars and boxes), at the start (BL RM ) during (RM), and 15 min following the three maneuvers: SLRM, PCRM, and ViCM. At 15 min after recruitment all three RMs resulted in increased PaO2 and compliance while shunt, alveolar dead space (ADS) and PCO2 decreased. The circulatory depression caused by the high-pressure RMs, ViCM>PCRM, was much less with the SLRM (p<0.01 between all). However, for all maneuvers circulation was largely restored within 5 min, and no significant differences remained after 15 min. *p<0.05 before vs. after lavage. Differences between maneuvers (p<0.05) are indicated during recruitment and 15 min after RM: a ViCM vs. PCRM, b ViCM vs. SLRM, and c PCRM vs. SLRM
Effect of recruitment procedures
PCRM and ViCM induced significantly greater maximal LV expansion during RMs (1089±76 and 1043±100 ml, respectively) than did SLRM (850±41 ml; p<0.01, p<0.05, respectively). However, 1 min after recruitment there was no difference in EELV increase between the RMs (Fig. 3). EELVN2 increased significantly from 407±31, 428±36, and 389±37 to 627±45, 644±56, and 612±53 ml 15 min following SLRM, PCRM, and ViCM, respectively (p<0.01 each). During SLRM tracheal plateau pressure was 27±0.9 cmH2O. Total respiratory compliance was unchanged during PCRM and did not increase until after the recruitment procedure. Compliance 1 min after ViCM was similar to that after PCRM. SLRM was more effective and a significant increase in compliance was observed during recruitment with further increase after release of the maneuver (p<0.01). Alveolar dead space increased following lavage from 9.4±0.2 to15.6±0.1% (p<0.01) and was equally reduced with all maneuvers to baseline values 15 min after recruitment. Shunt increased from 5±1% to 47±5% following lavage and was reduced during the RM to 14±4%, 12±2%, and 7±1% with ViCM, PCRM, and SLRM, respectively (p<0.05 vs. PCRM). After SLRM shunt remained low in contrast to PCRM and ViCM, where there was a transient increase after releasing maneuvers. All RMs improved oxygenation, but the increase in PaO2 during and after recruitment was greater with SLRM (p<0.01 vs. PCRM and ViCM). After release of RMs oxygenation transiently decreased, this drop was most pronounced after high-pressure RMs (Fig. 4). PaCO2 was significantly reduced 15 min after all RMs. Cardiac output was reduced during SLRM, PCRM, and ViCM by 21±3%, 44±2%, and 63±4% (p<0.01 between all RMs). Reduction in MAP was less pronounced. The decrease in SvO2 was most marked during the ViCM (Fig. 2). Mean pulmonary artery pressure increased during ViCM, decreased during SLRM, and was unchanged with PCRM. All RMs resulted in a similar reduction in mean pulmonary artery pressure 15 min after recruitment.
Lung volume changes (mean ±SEM) calculated from EIT measurements from end expiratory values at baseline prior to recruitment at PEEP 5 cmH2O during and after: slow low-pressure maneuver (SLRM, open bars), maneuver in pressure control ventilation (PRCM, striped bars), and vital capacity maneuver at PEEP 10 cmH2O (ViCM, gray bars). Absolute EELVN2 prior to recruitment was 407±31, 428±36, and 389±37 ml with SLRM, PCRM, and ViCM, respectively. RM MAX Maximal increase in lung volume, from EIT, at end inspiration during the three maneuvers. Note that although high-pressure maneuvers (PCRM and ViCM) induce greater lung volume expansion during the maneuver (†p<0.01 vs. PCRM, *p<0.05 vs. ViCM), there was no significant difference in end expiratory lung volume increase as early as 1 min after cessation of recruitment
Corresponding changes in lung volume (gray) assessed from EIT and in PaO2 (black) during three typical recruitment procedures in one pig. Forceful recruitment using high-pressure maneuvers (ViCM and PCRM) rapidly expands the lungs, but the long-lasting effect at 15 min after recruitment is similar to that using a slow, less traumatic maneuver (SLRM). Following release of maneuvers a drop in PaO2 is observed
Regional ventilation distribution
Lavage induced lung collapse in dorsal regions and redistribution of ventilation towards ventral parts. At baseline 32±2% of the tidal ventilation occurred in the two dorsal regions of the lung. This fraction was further reduced following lavage to 22±2%. Following lung expansion PEEP 10 cmH2O was sufficient to maintain LV in the two ventral regions while there was a gradual decrease in the dorsal regions in most animals, indicating recollapse (Fig. 5). Despite this, 15 min after RMs distribution of ventilation was similar to prelavage levels, being 36±2%, 35±2%, and 35±2% in dorsal regions after ViCM, PCRM, and SLRM, respectively.
The regional EIT tracings representing the ventral (V), midventral (MV), middorsal (MD), and dorsal (D) lung regions of the slice showed an increased EELV in all regions. The increase in lung volume detected by EIT in each region of interest during and after a vital capacity maneuver. With PEEP 10 cmH2O after recruitment lung volume in the two ventral regions remained stable while there was a gradual decrease in lung volume in the dorsal regions, indicating a recollapse
Regional compliance
In lung injured animals compliance in the ventral, midventral, middorsal, and dorsal regions was 3.6±0.3, 7.8±0.5, 2.8±0.4, and 0.6±0.1 ml/cmH2O, respectively. During SLRM and PCRM compliance in midventral parts was unchanged but increased significantly in dorsal regions (Fig. 6). With PCRM in contrast to SLRM compliance decreased significantly (p<0.01) in ventral parts.
Relative changes in regional compliance during SLRM and PCRM and 2, 5, and 15 min after all three maneuvers (SLRM, PCRM, and ViCM). Compliance in the middorsal and dorsal regions was significantly more increased during recruitment with an SLRM than with a PCRM. In the ventral region compliance actually decreased during a PCRM. Improvement in total respiratory compliance following all RMs was caused by significant increases in compliance in middle and dorsal regions, while compliance in the ventral region was unchanged. There was no difference 15 min after the RMs. *p<0.05 vs. SLRM, ‡p<0.01 vs. baseline before recruitment
Discussion
This study using EIT to evaluate the effect of two rapid high-pressure maneuvers (ViCM and PCRM) and one slow with lower pressure (SLRM). Improvement in LV, compliance, and gas exchange 15 min after recruitment was observed with all RMs, but circulatory depression during recruitment was significantly less using SLRM. During PCRM, in contrast to SLRM, there were signs of overdistention in ventral parts of the lung. Successful recruitment was associated with an increase in compliance in middle and dorsal regions and a redistribution of ventilation towards dependent lung areas. This study also indicates the potential usefulness of the EIT for monitoring of LV changes.
Methodological aspects
Depending on the cause of the lung injury various responses to ventilatory interventions can be expected [26, 27, 28]. The lavage model used in this study is characterized by surfactant depletion, atelectasis formation, increased extravascular lung water, and decreased compliance and is considered a stable experimental model of early acute respiratory distress syndrome (ARDS) [24, 25, 29]. However, RMs, PEEP and high TV have been shown to recruit collapsed lung areas and maintain LV more easily in lavage-induced ALI, in contrast to oleic acid and pneumonia-induced models [26, 27]. Therefore our results cannot be directly extrapolated to other experimental models or clinical situations primarily involving pulmonary edema or inflammation with secondary surfactant dysfunction.
Another limitation of this study is that LV changes and regional ventilation were assessed by analyzing lung impedance changes in only one slice (5–10 cm thick). The correlation of impedance changes with known LV changes using a super syringe allows the use of impedance changes in one slice during recruitment procedures even though the lungs may move during RMs. Concerning data obtained on regional ventilation and volumes conclusions can only be drawn on the slice measured, especially in inhomogeneous lungs such as in ALI/ARDS.
We observed that the effect of the RMs on EELV was greater measured with EIT than with a N2 washout/washin technique. This could possibly be explained by EELVN2 primarily measuring the “effective functional residual capacity” in terms of gas exchange while EIT measuring changes in electrical properties related to the total amount of air in relation to fluid in the scanned slice. Similar differences have been showed comparing measurements of functional residual capacity using SF6 and CT in ARDS patients [30].
Recruitment maneuvers
Pulmonary effects
This study shows that alveolar recruitment can be achieved using lower airway pressure levels maintained over a longer period. With the slow, lower pressure RM used in the present study LV initially increased rapidly, probably as an effect of PEEP elevation to 15 cmH2O, and the ensuing gradual increase throughout the 15 min of recruitment was probably a combined effect of repeated prolonged end-inspiratory pauses and PEEP elevation. Interestingly, these effects were achieved with ventilation using relatively low plateau pressure (27±0.9 cmH2O), which was maintained over a longer period than with high-pressure RMs. Previous studies have indicated that both airway pressure and time determines the effect of RMs [1]. The plateau pressure used in this study is in agreement with current protective ventilatory strategies avoiding pressures above 30 cmH2O [31], while recruitment strategies including ViCM, PCRM, or sighs [32, 33] use peak airway pressure of 40–50 cmH2O. Whether increased PEEP maintained for longer period alone can increase LV remains to be further investigated [34, 35]. The high-pressure RMs increased LV significantly more than SLRM during recruitment, but even 1 min after cessation of maneuvers the gain in EELV did not differ between RMs (Fig. 3).
However, the success of a RM cannot be judged from the increase in LV alone since this could be the result of the same number of alveoli being more inflated. It has been proposed that successful recruitment should result in an increase in compliance and oxygenation and a decrease in PaCO2. This study observed an increase in LV, PaO2 and compliance and a decrease in PaCO2 15 min after completion of recruitment for all three maneuvers. Interestingly, a slow low-pressure RM resulted in greater improvement in oxygenation, shunt, and compliance than the high-pressure maneuvers.
During the RMs total as well as midventral, middorsal, and dorsal regional compliance increased significantly and compliance in ventral parts remained unchanged, confirming that the increase in EELV is the result of recruitment and not overinflation. As a result of this redistribution of the TV to the middorsal and dorsal regions occurred. This was seen irrespective of RM. However, during PCRM compliance decreased with 50% in the ventral region as a sign of baro-/volostress to these alveoli (Fig. 6). This was not seen with SLRM. Thus it seems that the PCRM results in effective recruitment of alveoli but at the cost of overdistension, whereas the slow, low pressure RM lacks this negative effect. During the ViCM it was not possible to calculate regional compliance since this is a nonventilatory maneuver, but as the pressure used is of the same magnitude as during the PCRM, mechanical stress is probably similar.
Irrespective of RM it is important to maintain an increased PEEP following recruitment [35, 36, 37]. Dyhr et al. [35] showed that PEEP after the RM kept the lung open, but as soon as PEEP was decreased LV returned to the pre-RM level.
In the present study, despite a PEEP of 10 cmH2O after recruitment there was a gradual decrease in EELV, stabilizing at around 15 min. The regional EIT tracing showed no decrease in EELV in the ventral regions but a prominent decrease was seen in dorsal regions (Fig. 5). Thus the regions where most of the recruitment occurred were also those most prone to lose its recruited volume. It is possible that the decrease in LV in the two lower regions could have been prevented by a higher PEEP level, combined with a lower TV to avoid overdistension of the upper regions. Possibly, regional EIT could be used for postrecruitment optimal PEEP and TV titration.
Hemodynamic effects
In accordance with previous studies [4, 5, 6] we observed a marked decrease in cardiac output during the RMs: ViCM>PCRM>SLRM, where the modest hemodynamic effect of the SLRM probably is explained by the relatively low airway pressures. Following release of RMs a significant decrease in arterial oxygenation occurred. This was probably due to a return of “pooled” poorly saturated blood from the periphery while a pulmonary shunt of around 10% still remained. With SLRM this phenomenon was less pronounced (Figs. 2, 4).
Conclusion
In this porcine surfactant deficiency ALI model representing early ARDS lung recruitment was achieved with a slow, low pressure RM with less circulatory depression and probably less risk of barotrauma. This less aggressive RM actually resulted in greater improvement in oxygenation, shunt, and compliance than high-pressure maneuvers. LV changes could be monitored by EIT and regional ventilation distribution assessed. Although the EIT technique has limitations and needs further development, it could be valuable as a dynamic monitoring tool to optimize ventilatory care of ALI patients in the future.
References
Rothen HU, Neumann P, Berglund JE, Valtysson J, Magnusson A, Hedenstierna G (1999) Dynamics of re-expansion of atelectasis during general anaesthesia. Br J Anaesth 82:551–556
Lapinsky SE, Aubin M, Mehta S, Boiteau P, Slutsky AS (1999) Safety and efficacy of a sustained inflation for alveolar recruitment in adults with respiratory failure. Intensive Care Med 25:1297–1301
Fujino Y, Goddon S, Dolhnikoff M, Hess D, Amato MB, Kacmarek RM (2001) Repetitive high-pressure recruitment maneuvers required to maximally recruit lung in a sheep model of acute respiratory distress syndrome. Crit Care Med 29:1579–1586
Grasso S, Mascia L, Del Turco M, Malacarne P, Giunta F, Brochard L, Slutsky AS, Marco Ranieri V (2002) Effects of recruiting maneuvers in patients with acute respiratory distress syndrome ventilated with protective ventilatory strategy. Anesthesiology 96:795–802
Lim SC, Adams AB, Simonson DA, Dries DJ, Broccard AF, Hotchkiss JR, Marini JJ (2004) Transient hemodynamic effects of recruitment maneuvers in three experimental models of acute lung injury. Crit Care Med 32:2378–2384
Odenstedt H, Aneman A, Karason S, Stenqvist O, Lundin S (2005) Acute hemodynamic changes during lung recruitment in lavage and endotoxin-induced ALI. Intensive Care Med 31:112–120
Boussarsar M, Thierry G, Jaber S, Roudot-Thoraval F, Lemaire F, Brochard L (2002) Relationship between ventilatory settings and barotrauma in the acute respiratory distress syndrome. Intensive Care Med 28:406–413
Villagra A, Ochagavia A, Vatua S, Murias G, Del Mar Fernandez M, Lopez Aguilar J, Fernandez R, Blanch L (2002) Recruitment maneuvers during lung protective ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 165:165–170
Musch G, Harris RS, Vidal Melo MF, O’Neill KR, Layfield JD, Winkler T, Venegas JG (2004) Mechanism by which a sustained inflation can worsen oxygenation in acute lung injury. Anesthesiology 100:323–330
Odenstedt H, Lindgren S, Olegard C, Lethvall S, Söndergaard S, Stenqvist O, Lundin S (2004) Changes in lung volume during recruitment manoeuvres (RM) assessed by electric impedance tomography (EIT). Eur J Anaesthesiol 21 [Suppl 32]:A650
Odenstedt H, Lindgren S, Erlandsson K, Olegard C, Sondergaard S, Lundin S, Stenqvist O (2004) Lung recruitment assessed by reginal ventilation distribution using electric impedance tomography. Intensive Care Med 30 [Suppl 1]:A506
Patroniti N, Bellani G, Manfio A, Maggioni E, Giuffrida A, Foti G, Pesenti A (2004) Lung volume in mechanically ventilated patients: measurement by simplified helium dilution compared to quantitative CT scan. Intensive Care Med 30:282–289
Wrigge H, Sydow M, Zinserling J, Neumann P, Hinz J, Burchardi H (1998) Determination of functional residual capacity (FRC) by multibreath nitrogen washout in a lung model and in mechanically ventilated patients. Accuracy depends on continuous dynamic compensation for changes of gas sampling delay time. Intensive Care Med 24:487–493
Jonmarker C, Jansson L, Jonson B, Larsson A, Werner O (1985) Measurement of functional residual capacity by sulfur hexafluoride washout. Anesthesiology 63:89–95
Malbouisson LM, Muller JC, Constantin JM, Lu Q, Puybasset L, Rouby JJ (2001) Computed tomography assessment of positive end-expiratory pressure-induced alveolar recruitment in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 163:1444–1450
Adler A, Amyot R, Guardo R, Bates JH, Berthiaume Y (1997) Monitoring changes in lung air and liquid volumes with electrical impedance tomography. J Appl Physiol 83:1762–1767
Frerichs I, Hinz J, Herrmann P, Weisser G, Hahn G, Dudykevych T, Quintel M, Hellige G (2002) Detection of local lung air content by electrical impedance tomography compared with electron beam CT. J Appl Physiol 93:660–666
Hinz J, Neumann P, Dudykevych T, Andersson LG, Wrigge H, Burchardi H, Hedenstierna G (2003) Regional ventilation by electrical impedance tomography: a comparison with ventilation scintigraphy in pigs. Chest 124:314–322
Victorino JA, Borges JB, Okamoto VN, Matos GF, Tucci MR, Caramez MP, Tanaka H, Sipmann FS, Santos DC, Barbas CS, Carvalho CR, Amato MB (2004) Imbalances in regional lung ventilation: a validation study on electrical impedance tomography. Am J Respir Crit Care Med 169:791–800
Karason S, Sondergaard S, Lundin S, Wiklund J, Stenqvist O (2000) Evaluation of pressure/volume loops based on intratracheal pressure measurements during dynamic conditions. Acta Anaesthesiol Scand 44:571–577
Olegård C, Söndergaard S, Lundin S, Houltz E, Stenqvist O (2005) Estimation of functional residual capacity (FRC) at the bedside using standard monitoring equipment: modified nitrogen washout/washin technique requiring a small change of the inspired oxygen fraction. Anesth Analg (in press)
Hahn GD, Frerichs T, Thiel I, Hellige FG (2002) A high performance electric impedance tomography (EIT) system for clinical evaluation studies and space application. In: Proceedings of the 2nd European Medical & Biological Engineering Conference. TU Graz, Graz
Frerichs I, Hahn G, Hellige G (1999) Thoracic electrical impedance tomographic measurements during volume controlled ventilation-effects of tidal volume and positive end-expiratory pressure. IEEE Trans Med Imaging 18:764–773
Lachmann B, Robertson B, Vogel J (1980) In vivo lung lavage as an experimental model of the respiratory distress syndrome. Acta Anaesthesiol Scand 24:231–236
Nielsen JB, Sjostrand UH, Edgren EL, Lichtwarck-Aschoff M, Svensson BA (1991) An experimental study of different ventilatory modes in piglets in severe respiratory distress induced by surfactant depletion. Intensive Care Med 17:225–233
Neumann P, Berglund JE, Mondejar EF, Magnusson A, Hedenstierna G (1998) Dynamics of lung collapse and recruitment during prolonged breathing in porcine lung injury. J Appl Physiol 85:1533–1543
Kloot TE, Blanch L, Melynne Youngblood A, Weinert C, Adams AB, Marini JJ, Shapiro RS, Nahum A (2000) Recruitment maneuvers in three experimental models of acute lung injury. Effect on lung volume and gas exchange. Am J Respir Crit Care Med 161:1485–1494
Lim SC, Adams AB, Simonson DA, Dries DJ, Broccard AF, Hotchkiss JR, Marini JJ (2004) Intercomparison of recruitment maneuver efficacy in three models of acute lung injury. Crit Care Med 32:2371–2377
Lichtwarck-Aschoff M, Mols G, Hedlund AJ, Kessler V, Markstrom AM, Guttmann J, Hedenstierna G, Sjostrand UH (2000) Compliance is nonlinear over tidal volume irrespective of positive end-expiratory pressure level in surfactant-depleted piglets. Am J Respir Crit Care Med 162:2125–2133
Rylander C, Tylen U, Rossi-Norrlund R, Herrmann P, Quintel M, Bake B (2005) Uneven distribution of ventilation in acute respiratory distress syndrome. Crit Care 9:R165–R171
The Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Foti G, Cereda M, Sparacino ME, De Marchi L, Villa F, Pesenti A (2000) Effects of periodic lung recruitment maneuvers on gas exchange and respiratory mechanics in mechanically ventilated acute respiratory distress syndrome (ARDS) patients. Intensive Care Med 26:501–507
Pelosi P, Bottino N, Chiumello D, Caironi P, Panigada M, Gamberoni C, Colombo G, Bigatello LM, Gattinoni L (2003) Sigh in supine and prone position during acute respiratory distress syndrome. Am J Respir Crit Care Med 167:521–527
Richard JC, Brochard L, Vandelet P, Breton L, Maggiore SM, Jonson B, Clabault K, Leroy J, Bonmarchand G (2003) Respective effects of end-expiratory and end-inspiratory pressures on alveolar recruitment in acute lung injury. Crit Care Med 31:89–92
Dyhr T, Nygard E, Laursen N, Larsson A (2004) Both lung recruitment maneuver and PEEP are needed to increase oxygenation and lung volume after cardiac surgery. Acta Anaesthesiol Scand 48:187–197
Halter JM, Steinberg JM, Schiller HJ, DaSilva M, Gatto LA, Landas S, Nieman GF (2003) Positive end-expiratory pressure after a recruitment maneuver prevents both alveolar collapse and recruitment/derecruitment. Am J Respir Crit Care Med 167:1620–1626
Lim CM, Jung H, Koh Y, Lee JS, Shim TS, Lee SD, Kim WS, Kim DS, Kim WD (2003) Effect of alveolar recruitment maneuver in early acute respiratory distress syndrome according to antiderecruitment strategy, etiological category of diffuse lung injury, and body position of the patient. Crit Care Med 31:411–418
Acknowledgements
The authors thank Dr. Annette Nyberg and Marita Ahlqvist EMA for valuable assistance during the experiments. The EIT equipment was supplied by Dräger Medical AG, Germany, and the authors are grateful for valuable advice from Mr. Eckhard Teschner. This study was supported by grants from the Medical College of Gothenburg University, the Swedish Research Council (K2003-04X-14032-03A), and the Gothenburg Medical Society. The results of this study were in part presented at the ESA meeting in Lisbon 2004 and the ESICM meeting in Berlin, 2004.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: http://dx.doi.org/10.1007/s00134-005-2800-4
Rights and permissions
About this article
Cite this article
Odenstedt, H., Lindgren, S., Olegård, C. et al. Slow moderate pressure recruitment maneuver minimizes negative circulatory and lung mechanic side effects: evaluation of recruitment maneuvers using electric impedance tomography. Intensive Care Med 31, 1706–1714 (2005). https://doi.org/10.1007/s00134-005-2799-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2799-6