Abstract
Objective
The objective was to analyse relationships between inspiratory and expiratory static and dynamic elastic pressure-volume (Pel/V) curves in healthy pigs.
Design
The modulated low flow method was developed to allow studies also of the expiratory Pel/V curves. Static Pel/V (Pel,st/V) and dynamic Pel/V (Pel,dyn/V) loops were studied in healthy pigs.
Setting
Animal research laboratory in a university hospital.
Material
Ten healthy anaesthetised and paralysed pigs.
Interventions and measurements
A computer controlled a Servo Ventilator 900C with respect to respiratory rate, inspiratory flow and expiratory pressure to achieve a sinusoidal modulation of inspiration and expiration for determination of Pel,dyn/V loops from zero end-expiratory pressure (ZEEP) and from a positive end-expiratory pressure (PEEP) of 6 cmH2O to 20, 35 and 50 cmH2O. The same system was used for studies of Pel,st/V loops with the flow-interruption method from ZEEP and PEEP to 35 cmH2O. Recordings were analysed with an iterative technique.
Results
The feasibility of automated determination of Pel,dyn/V loops was demonstrated. Differences between Pel,dyn/V and Pel,st/V loops were explained by viscoelastic behaviour. Pel,st/V loops recorded from PEEP to 35 cmH2O showed no significant hysteresis, indicating a non-significant surface tension hysteresis. Pel,dyn/V loops from PEEP and both Pel,st/V and Pel,dyn/V loops from ZEEP to 35 cmH2O showed hysteresis. This indicates that lung collapse/re-expansion caused Pel/V loop hysteresis which, in Pel,dyn/V loops, was augmented by viscoelastic behaviour.
Conclusions
Viscoelasticity influences Pel,dyn/V curves. Hysteresis caused by surface tension merits re-evaluation. Lung collapse and re-expansion may be indicated by hysteresis of Pel/V loops.
Similar content being viewed by others
Introduction
The inspiratory elastic pressure-volume (Pel/V) curve recorded from zero end-expiratory pressure (ZEEP) may be used as a guideline for ventilator setting in acute lung injury (ALI) [1, 2]. However, recent studies illustrate difficulties in interpreting the Pel/V curve [3, 4, 5, 6]. Multiple inspiratory curves recorded from different levels of PEEP and from ZEEP provide more detailed information about pressures at which lung de-recruitment and recruitment occurs [7, 8, 9]. An alternative is to record loops comprising inspiratory and expiratory Pel/V curves [10, 11, 12].
The super-syringe method and the flow-interruption technique allow determination of static Pel/V (Pel,st/V) loops, but these methods have important limitations such as time consumption. Dynamic Pel/V (Pel,dyn/V) curves can be determined in a few seconds using moderately low flow rates. In order to separate flow-dependent resistive pressure from volume-dependent elastic pressure, one must vary both flow rate and volume. For static curves this is achieved by stopping the flow at different volume intervals. For dynamic curves it has been shown that sinusoidal flow modulation can be used for the purpose of simultaneous determination of resistance and the Pel,dyn/V curve [7, 13]. Hence, in a clinical setting the inspiratory Pel,dyn/V curve was determined during a 6-s-long sinusoidal insufflation, meeting the requirements for a fast clinical method. Mathematical separation between elastic and resistive pressures was achieved according to the principle of least sum of squared differences between measured tracheal pressure and tracheal pressure expressed by an equation summing elastic and resistive pressures.
The relationship between Pel,st/V and Pel,dyn/V curves has been studied only for inspiration [14, 15]. The objective of this study was to analyse the relationships between inspiratory and expiratory Pel,st/V and Pel,dyn/V curves in healthy pigs in order to increase the understanding of the different nature of these curves and the physiology behind hysteresis of the loops. For this purpose the modulated low flow method was further developed to allow recording and analysis of both inspiratory and expiratory limbs of Pel,dyn/V loops recorded at different PEEP levels.
Materials and methods
Animal model
Ten healthy pigs (17.0–21.4 kg) were pre-medicated with azaperon (Stresnil, Jansen, Beerse, Belgium), 7 mg kg−1, anaesthetised with intravenous (i.v.) ketamine (Ketalar, Parke-Davis, Morris Plains, USA), 5 mg kg−1, intubated with a 6.5 mm ID tracheal tube and connected to a ventilator (Servo Ventilator 900C, Siemens-Elema, Sweden). Anaesthesia was maintained by continuous i.v. infusion of ketamine 17 mg kg−1 h−1 and midazolam (Dormicum, Hoffmann-La Roche, Basel, Switzerland) 1.7 mg kg−1 h−1. Muscle relaxation was obtained by i.v. pancuronium bromide (Pavulon, Organon Teknika, Boxtel, Holland) 0.5 mg kg−1 h−1. Ventilation was volume-controlled at a PEEP level of 6 cmH2O. Respiratory rate (RR) was 20 breaths min−1. Inspiration and the post-inspiratory pause lasted 33 and 5% of the respiratory cycle, respectively. Minute volume (MV) was adjusted to an end tidal PCO2 of 4.5−5.0 kPa. Heart rate and arterial blood pressure were monitored using a femoral artery catheter.
Procedure
After stabilisation for 1 h, a leak test was performed during a prolonged post-inspiratory pause. At least two dynamic pressure-volume (P/V) loops were recorded for determination of the resistance of the respiratory system and determination of the inspiratory volume needed to reach 50 cmH2O during insufflation from ZEEP. A recruitment manoeuvre before each recording comprised three insufflations to 45 cmH2O maintained for 15 s, interposed by 4-s-long expirations at a PEEP of 15 cmH2O.
Dynamic elastic pressure-volume loops were recorded for six different pressure intervals, i.e. from zero and from 6 cmH2O to 20, 35 and 50 cm H2O, in random order. Pel,st/V loops were recorded for two different pressure intervals, i.e. from zero and from 6 cmH2O to 35 cmH2O. Sets of static and dynamic loops were recorded in random order. Within the static set, the order of inspiratory and expiratory curves was randomised, so was the order of PEEP and ZEEP loops. For Pel,dyn/V loops the expiratory limb was always recorded immediately following the inspiratory limb, as described below.
After the experiments the pigs were killed. The local ethics board of animal research approved the protocol.
Recordings and analysis
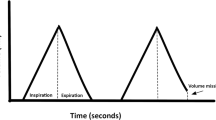
The modulated low flow technique [7, 13] was amended by sinusoidal flow modulation, also during the expiration following the flow-modulated inspiration. The operator specified the sequence of breaths comprising a P/V loop recording. A normal breath was followed by a breath with a 6-s-long expiration at ZEEP or 6 cmH2O, below denoted as ZEEP and PEEP (Fig. 1). The following sinusoidal inspiration continued until a pressure of 20, 35 or 50 cmH2O was reached. After a 0.9-s end-inspiratory pause a prolonged sinusoidal expiration continued until the starting PEEP level was reached. To achieve this sequence of breaths, the ventilator was electronically controlled by a personal computer as described in the electronic supplement [7, 13]. In short, signals emitted from the computer controlled MV and RR to modify the inspiration with respect to duration and flow rate. The inspiratory pause was prolonged by controlling RR. The computer controlled PEEP during the preceding expiration. During the succeeding modulated expiration the PEEP level was ‘stepwise’ reduced so that a nearly sinusoidal flow pattern was achieved. The signals from the ventilator representing pressure in the expiratory line (P) and flow rate were sampled at a rate of 50 s−1.
The principles for analysis of the dynamic recordings have been published [13]. Further details are described in the electronic supplement. To calculate the airway flow rate (V̇), measured flow was corrected for gas compression in the ventilator tubing. The pressure, below denoted ‘measured’ tracheal pressure, was obtained by subtracting the resistive pressure drop from the Y-piece (site of pressure measurement) to the tracheal end of the endotracheal tube. Tracheal pressure (Ptr) comprises the resistive pressure of the respiratory system (Pres,RS) and its dynamic elastic recoil pressure (Pel,dyn).
R is the resistance of the respiratory system that may vary with lung volume.
Inspiratory resistance was considered constant, i.e. R1 was zero. During expiration, R1 was zero or negative, reflecting the fact that expiratory resistance might significantly increase towards lower lung volume.
Static and dynamic Pel/V curves can be characterised as a non-symmetric sigmoid with a middle linear segment over which compliance is constant (CLIN) [4, 16]. Below the lower inflection point with its co-ordinates (LIP, VLIP, PLIP) and above the upper inflection point (UIP, VUIP, PUIP) compliance falls linearly in relation to volume. Compliance would approach a value of zero when the volume decreases towards a minimal volume (Vmin) and increases towards a maximal volume (Vmax). Vmin and Vmax are the asymptotes of the extrapolated Pel/V curve. The Pel/V curve is mathematically described by the six coefficients CLIN, VLIP, PLIP, VUIP, Vmin and Vmax.
Estimation of the seven or, for expiration, eight coefficients describing Pel,dyn and R in relation to volume was achieved by finding values giving the minimum sum of squared differences between the measured Ptr and the calculated Ptr described by Equation 1. This was done by an iterative technique using the Solver of Excel 97 (Microsoft Excel 97, Microsoft, Redmond, WA, US). All the data presented represent the parameterised results.
Hysteresis index, which describes the area of the loop in relation to a rectangle circumscribing the loop, was calculated according to Bachofen et al. [17]. The volume difference between the inspiratory and expiratory limbs of the Pel,dyn/V loop is denoted ΔVhyst. ΔVhyst, inspiratory and expiratory compliance were calculated over the whole pressure range.
The Pel,dyn/V loops recorded from ZEEP and PEEP at each condition were aligned at the end-expiratory volume of the ordinary breath preceding the measurement. The volume scale of both curves refers to the elastic equilibrium volume reached after the prolonged expiration at ZEEP.
Inspiratory and expiratory Pel,st/V loops were recorded from ZEEP and from a PEEP of 6 cmH2O to 35 cmH2O according to the flow-interruption technique [18]. This measurement was automatic under computer control, as previously described for inspiratory curves [19]. For curves from PEEP and ZEEP 15 and 20 interrupted breaths, respectively, were used for each of the inspiratory and expiratory Pel,st/V curves. In order to suppress heart artefacts, the static elastic recoil pressure (Pel,st) was measured as the mean pressure over a complete heart cycle 3 s after flow interruption. The mathematical analysis of the Pel,st/V curve was the same as for the Pel,dyn/V curves.
Statistics
The results are presented as the average values ± SD, or average values ± SEM when interest is focused on differences between groups. Paired comparisons were made with t-test. 2-way ANOVA was used when indicated. Probability values lower than 0.05 indicated significant differences.
Results
Adequate sinusoidal flow modulation was as previously [7, 13] achieved during volume-controlled inspiration (Fig. 1). However, during late expiration the amplitude of the flow modulation decreased, so that zero flow was not reached.
Resistance
The sinusoidal pressure oscillations superimposed on the elastic pressure were in general less than 1 cmH2O. This corresponded to values of inspiratory resistance of 1.9±0.8 cmH2O/(l/s) and values of resistance in the middle of expiration of 2.5±1.2 cmH2O/(l/s), measured during sinusoidal inspirations and expirations to and from 35 cmH2O, respectively.
Elastic pressure-volume loops
The curvilinearity of Pel,dyn/V curves recorded both from PEEP and from ZEEP to 35 cmH2O appeared larger than that of corresponding Pel,st/V curves (Fig. 2A and 2B). This was verified by the fact that dynamic compliance (Cdyn) varied significantly more with Pel than static compliance (Cst) for inspiratory and expiratory ZEEP curves and expiratory PEEP curves (p<0.004). Furthermore, initially during both inspiration and expiration Cdyn was lower than Cst for both ZEEP and PEEP curves (p<0.05). For ZEEP curves Cdyn fell to significantly lower values than Cst during the second part of inspiration. As a consequence of more linear static curves, an inspiratory LIP was barely discernible in most pigs. The vaguely perceptible inspiratory LIP in static curves occurred at lower pressures than the more evident inspiratory LIP in dynamic curves (Table 1). Static and dynamic CLIN differed slightly (Table 1).
Averaged Pel,st/V loops from PEEP showed no hysteresis (Fig. 2A). The inspiratory and expiratory limbs had the same shape, as shown by similar compliance at equal Pel (Fig. 2C). The Pel,st/V loop from ZEEP and the Pel,dyn/V loops from ZEEP and PEEP to 35 cmH2O showed hysteresis (Fig. 2A and 2B). For all these loops expiratory compliance was significantly lower than inspiratory at the beginning of expiration and significantly higher during the later part of expiration (Fig. 2D, 2E and 2F).
In Pel,dyn/V loops from ZEEP to 50 cmH2O the inspiratory limbs showed well-known features with lower and upper inflection points (Fig. 3A, Table 2). The expiratory limb started with a segment with low compliance followed by a linear segment below the UIP. The numerical analysis identified an expiratory LIP in a single pig. This was visually non-distinct. Expiratory CLIN was higher than inspiratory (p<0.001).
In Pel,dyn/V loops from PEEP to 50 cmH2O inspiratory PLIP and PUIP were consistently higher than in loops from ZEEP (p<0.001), while CLIN was similar. For loops starting at ZEEP and at PEEP, the expiratory limbs coincided and showed similar PUIP and CLIN (Fig. 3A, Table 2).
Inspiratory Pel,dyn/V curves recorded from ZEEP to 20, 35 and 50 cmH2O started from the same situation. Consequently, they overlapped. At equal Pel, volumes of the ensuing expiratory curves were initially significantly higher for curves starting at higher pressures (Fig. 3B) and these curves showed higher CLIN. Thus, for expiratory Pel,dyn/V curves starting at 50, 35 and 20 cmH2O, CLIN was 55±9, 49±11 and 44±8 ml/cmH2O, respectively (p<0.001, ANOVA). For loops starting at PEEP the pattern was similar although differences in CLIN were not significant.
Over the whole common pressure interval, inspiratory Pel,dyn/V curves recorded from ZEEP to 20, 35 and 50 cmH2O were displaced towards significantly lower volumes than curves starting at PEEP (p<0.001 for pressures up to 40 cmH2O) which, for the latter curves, is shown in Fig. 3A. During the following expirations the difference between the ZEEP and PEEP curves decreased to become non-significant below 30 cmH2O for expirations starting at 50 cmH2O and below 11 cmH2O for expirations starting at 20 and 35 cmH2O.
Hysteresis
Hysteresis of Pel,dyn/V loops, expressed as ΔVhyst, increased with the size of the pressure interval. The difference between ΔVhyst in Pel,dyn/V loops to 35 and 50 cmH2O was significant (Fig. 4). Pel,dyn/V loops from PEEP to 50 cmH2O showed significantly smaller ΔVhyst up to a pressure of 43 cmH2O compared to loops from ZEEP.
The average volume difference between the inspiratory and expiratory limbs (ΔV hyst ) ± SEM in dynamic elastic pressure-volume (Pel,dyn/V) loops starting at zero end-expiratory pressure (ZEEP) and positive end-expiratory pressure (PEEP) (left and right panels, respectively). Differences between the ΔVhyst curves were significant over the segments where SEM curves did not overlap
Hysteresis index for Pel,dyn/V loops from ZEEP and PEEP, covering the three different pressure ranges, were similar and on average 0.075±0.023.
Discussion
Early studies in intensive care and mechanical ventilation were based on Pel/V loops derived by the super-syringe method [20]. However, these methods have important limitations, particularly the need for disconnection from the ventilator and errors induced by continuing gas exchange [21, 22]. Later studies of the static pressure-volume relationship of the lungs in mechanical ventilation have often focused on inspiratory Pel/V curves derived by more and more automated multiple occlusion methods [14, 18, 19, 23, 24]. The low flow inflation method has been developed going from constant flow to sinusoidal flow [7, 13, 14, 25]. The modulated flow provides simultaneous data of resistance and, therefore, more accurate Pel/V curves [13]. An inspiratory Pel/V curve recorded from ZEEP is difficult to interpret since it reflects continuing re-expansion over a large pressure interval. The latest development has been automatic analysis of multiple inspiratory Pel/V curves [8, 9]. These studies together with even later studies demonstrate that multiple inspiratory Pel/V curves give valuable information with respect to lung collapse and recruitment at different PEEP levels [26, 27]. However, the recording of multiple Pel/V curves takes some minutes. It is conceivable that the recording of Pel/V loops may give similar information in a fraction of that time.
As early as 1957 Mead et al. showed that studies of Pel/V loops and hysteresis give information about irregular expansion of a collapsed lung [28]. Recent experimental studies show that such loops illustrate features of lung collapse and re-expansion [10, 11, 12]. Accordingly, Pel/V loops remain interesting if they can be made automatically in a critical care environment.
In this material, resistance and the associated resistive pressure was so low that errors in its determination cannot induce significant errors in the Pel,dyn/V curves, which can be appreciated from Fig. E2 and E3 in the electronic supplement. However, when resistance is high adequate subtraction of the resistive pressure is important. We have abstained from reporting detailed data on resistance because we realise the difficulties in determining very low resistance values with precision. Our data regarding resistance are in line with previous observations that resistance in healthy beings is constant during inspiration, while increasing during expiration towards lower lung volumes [24]. Validation of the present method should be complemented in subjects with airway obstruction. The technical limitations observed with respect to expiratory flow regulation also need to be addressed, as further discussed in the electronic supplement.
We observed that the sinusoidal flow oscillations caused synchronous variations in pressure (Fig. 1 and Fig. E3 in the electronic supplement). Heart artefacts did not disturb the pattern of synchronous variations in flow rate and pressure. This is another issue which merits attention in clinical studies, as patients with critical lung disease often have enlarged heart artefacts [29].
The limbs of the Pel,dyn/V loops are recorded in immediate succession, which implies that their alignment is not a problem. The inspiratory and expiratory Pel,st/V curves, on the contrary, are recorded separately. Each comprised a large number of study breaths. The volume alignment in individual pigs was therefore less accurate, as evidenced by the fact that the volume at the start of expiration did not always coincide with that at the end of inspiration. Because of this static hysteresis was not quantified.
Hysteresis as observed in Fig. 2A and 2B might, in principle, reflect surface tension hysteresis, lung collapse/re-expansion and viscoelastic properties. Errors in the determination of resistance will be associated with errors in hysteresis. Important random errors would have obscured the differences observed in Fig. 2B and 2D. Conceivable systematic errors are not considered to affect the estimation of hysteresis, as further analysed in the electronic supplement. The Pel,st/V loop from PEEP to 35 cmH2O indicated no hysteresis (Fig. 2A). Furthermore, static inspiratory and expiratory limbs had the same shape, as illustrated by similar compliance over the whole pressure interval (Fig. 2C). This indicates that the Pel,st/V loops were influenced neither by surface tension hysteresis nor lung collapse/re-expansion when recorded from PEEP. This agrees with previous observations, e.g. early observations in healthy subjects by Mead et al. showed trivial hysteresis for breaths starting from normal FRC [28]. Furthermore, in anaesthetised humans, hysteresis is non-significant for normal tidal volumes [24]. Svantesson et al. developed the technique for Pel,st/V recording to avoid foreseeable errors, but still could not identify static hysteresis in healthy rabbits ventilated with PEEP [30]. The concept that the alveolar surfactant film contributes to hysteresis at tidal ventilation in health needs to be re-evaluated.
In contrast to Pel,st/V loops, Pel,dyn/V loops from PEEP showed hysteresis and corresponding differences in inspiratory and expiratory compliance (Fig. 2A and 2E). Viscoelastic behaviour offers an explanation of this finding as well as the observed larger curvilinearity of Pel,dyn/V curves compared to Pel,st/V curves. During the initial part of dynamic lung inflation or expiration at constant flow a viscoelastic pressure builds up until it reaches a steady state level [23, 31]. In simulation experiments we have found that this level is nearly stable even at sinusoidal flow modulation at 1.7 Hz (unpublished data). During the build-up of viscoelastic pressure Cdyn is lower than Cst, as was found in the beginning of both inspiration and expiration. Cessation of viscoelastic pressure build-up during inspiration may contribute to the better-defined LIP that occurs at a higher pressure in dynamic recordings compared to static ones. Another difference between Pel,st/V and Pel,dyn/V curves was that Cdyn fell more than Cst towards the end of insufflation to 35 cmH2O. A possible explanation is that a zone of non-linear viscoelastic properties was reached. In rabbits, Svantesson et al. found a pronounced non-linearity of viscoelastic properties at a high degree of lung distension leading to a fast build-up of viscoelastic pressure at the end of insufflation [30]. Then, the steady state level is interrupted and viscoelastic pressure increases further.
In recordings from ZEEP, even Pel,st/V loops showed hysteresis and significant differences between inspiratory and expiratory compliance (Fig. 2B and 2D). This may be explained by lung collapse/re-expansion. The contribution of lung re-expansion to compliance has long been known [32] and has recently been elucidated in theoretical, experimental and clinical studies [3, 4, 7, 8, 9]. The larger differences in Cdyn, compared to Cst, in Pel/V curves from ZEEP is in line with the summation of effects caused by viscoelastic forces and lung collapse/re-expansion (Fig. 2D and 2F). Earlier studies have shown that lungs may partly collapse after a single expiration to ZEEP in humans, but still more in pigs [33, 34, 35].
In conclusion, under controlled mechanical ventilation, sinusoidal flow modulation allows automated recording and detailed characterisation of Pel,dyn/V loops. Differences between Pel,dyn/V and Pel,st/V loops were explained by the more complex nature of Pel,dyn/V loops, mostly referable to viscoelastic behaviour. A non-significant hysteresis of Pel,st/V loops from PEEP is in line with previous observations that the alveolar surfactant film might not contribute to hysteresis. A significant hysteresis of Pel,st/V loops from ZEEP illustrates that lung collapse/re-expansion causes hysteresis. The prominent hysteresis of Pel,dyn/V loops from ZEEP reflects the combination of lung collapse/re-expansion and viscoelastic behaviour. Further validation of the method is needed, particularly with respect to potential problems related to airway obstruction.
References
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338:347–354
Roupie E, Dambrosio M, Servillo G, Mentec H, el Atrous S, Beydon L, Brun-Buisson C, Lemaire F, Brochard L (1995) Titration of tidal volume and induced hypercapnia in acute respiratory distress syndrome. Am J Respir Crit Care Med 152:121–128
Hickling KG (1998) The pressure-volume curve is greatly modified by recruitment. A mathematical model of ARDS lungs. Am J Respir Crit Care Med 158:194–202
Jonson B, Svantesson C (1999) Elastic pressure-volume curves: what information do they convey? Thorax 54:82–87
Pelosi P, Goldner M, McKibben A, Adams A, Eccher G, Caironi P, Losappio S, Gattinoni L, Marini JJ (2001) Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med 164:122–130
Crotti S, Mascheroni D, Caironi P, Pelosi P, Ronzoni G, Mondino M, Marini JJ, Gattinoni L (2001) Recruitment and derecruitment during acute respiratory failure: a clinical study. Am J Respir Crit Care Med 164:131–140
Jonson B, Richard JC, Straus C, Mancebo J, Lemaire F, Brochard L (1999) Pressure-volume curves and compliance in acute lung injury: evidence of recruitment above the lower inflection point. Am J Respir Crit Care Med 159:1172–1178
De Robertis E, Servillo G, Tufano R, Jonson B (2001) Aspiration of dead space allows isocapnic low tidal volume ventilation in acute lung injury. Relationships to gas exchange and mechanics. Intensive Care Med 27:1496–1503
Maggiore SM, Jonson B, Richard JC, Jaber S, Lemaire F, Brochard L (2001) Alveolar derecruitment at decremental positive end-expiratory pressure levels in acute lung injury. Comparison with the lower inflection point, oxygenation and compliance. Am J Respir Crit Care Med 164:795–801
Rimensberger PC, Cox PN, Frndova H, Bryan AC (1999) The open lung during small tidal volume ventilation: concepts of recruitment and “optimal” positive end-expiratory pressure. Crit Care Med 27:1946–1952
Rimensberger PC, Pristine G, Mullen BM, Cox PN, Slutsky AS (1999) Lung recruitment during small tidal volume ventilation allows minimal positive end-expiratory pressure without augmenting lung injury. Crit Care Med 27:1940–1945
Ingimarsson J, Björklund LJ, Larsson A, Werner O (2001) The pressure at the lower inflexion point has no relation to airway collapse in surfactant-treated premature lambs. Acta Anaesthesiol Scand 45:690–695
Svantesson C, Drefeldt B, Sigurdsson S, Larsson A, Brochard L, Jonson B (1999) A single computer-controlled mechanical insufflation allows determination of the pressure-volume relationship of the respiratory system. J Clin Monit 15:9–16
Servillo G, Svantesson C, Beydon L, Roupie E, Brochard L, Lemaire F, Jonson B (1997) Pressure-volume curves in acute respiratory failure: automated low flow inflation versus occlusion. Am J Respir Crit Care Med 155:1629–1636
Ranieri VM, Giuliani R, Fiore T, Dambrosio M, Milic-Emili J (1994) Volume-pressure curve of the respiratory system predicts effects of PEEP in ARDS: “occlusion” versus “constant flow” technique. Am J Respir Crit Care Med 149:19–27
Svantesson C, Sigurdsson S, Larsson A, Jonson B (1998) Effects of recruitment of collapsed lung units on the elastic pressure-volume relationship in anaesthetised healthy adults. Acta Anaesthesiol Scand 42:1149–1156
Bachofen H, Hildebrandt J (1971) Area analysis of pressure-volume hysteresis in mammalian lungs. J Appl Physiol 30:493–497
Levy P, Similowski T, Corbeil C, Albala M, Pariente R, Milic-Emili J, Jonson B (1989) A method for studying the static volume-pressure curves of the respiratory system during mechanical ventilation. J Crit Care 4:83–89
Svantesson C, Drefeldt B, Jonson B (1997) The static pressure-volume relationship of the respiratory system determined with a computer-controlled ventilator. Clin Physiol 17:419–430
Matamis D, Lemaire F, Harf A, Brun-Buisson C, Ansquer JC, Atlan G (1984) Total respiratory pressure-volume curves in the adult respiratory distress syndrome. Chest 86:58–66
Gattinoni L, Mascheroni D, Basilico E, Foti G, Pesenti A, Avalli L (1987) Volume/pressure curve of total respiratory system in paralysed patients: artefacts and correction factors. Intensive Care Med 13:19–25
Dall’ava-Santucci J, Armaganidis A, Brunet F, Dhainaut JF, Chelucci GL, Monsallier JF, Lockhart A (1988) Causes of error of respiratory pressure-volume curves in paralyzed subjects. J Appl Physiol 64:42–49
Similowski T, Levy P, Corbeil C, Albala M, Pariente R, Derenne JP, Bates JH, Jonson B, Milic-Emili J (1989) Viscoelastic behavior of lung and chest wall in dogs determined by flow interruption. J Appl Physiol 67:2219–2229
Jonson B, Beydon L, Brauer K, Månsson C, Valind S, Grytzell H (1993) Mechanics of respiratory system in healthy anesthetized humans with emphasis on viscoelastic properties. J Appl Physiol 75:132–140
Mankikian B, Lemaire F, Benito S, Brun-Buisson C, Harf A, Maillot JP, Becker J (1983) A new device for measurement of pulmonary pressure-volume curves in patients on mechanical ventilation. Crit Care Med 11:897–901
Richard JC, Maggiore SM, Jonson B, Mancebo J, Lemaire F, Brochard L (2001) Influence of tidal volume on alveolar recruitment. Respective role of PEEP and a recruitment maneuver. Am J Respir Crit Care Med 163:1609–1613
Richard JC, Brochard L, Vandelet P, Breton L, Maggiore SM, Jonson B, Clabault C, Leroy J, Bonmarchand G (2003) Respective effects of end-expiratory and end-inspiratory pressures on alveolar recruitment in acute lung injury. Crit Care Med 30:89–92
Mead J, Whittenberger JL, Radford JEP (1957) Surface tension as a factor in pulmonary volume-pressure hysteresis. J Appl Physiol 10:191–196
Beydon L, Svantesson C, Brauer K, Lemaire F, Jonson B (1996) Respiratory mechanics in patients ventilated for critical lung disease. Eur Respir J 9:262–273
Svantesson C, John J, Taskar V, Evander E, Jonson B (1996) Respiratory mechanics in rabbits ventilated with different tidal volumes. Respir Physiol 106:307–316
Bates JH, Ludwig MS, Sly PD, Brown K, Martin JG, Fredberg JJ (1988) Interrupter resistance elucidated by alveolar pressure measurement in open-chest normal dogs. J Appl Physiol 65:408–414
Frazer DG, Franz GN (1981) Trapped gas and lung hysteresis. Respir Physiol 46:237–246
Liu JM, De Robertis E, Blomquist S, Dahm PL, Svantesson C, Jonson B (1999) Elastic pressure-volume curves of the respiratory system reveal a high tendency to lung collapse in young pigs. Intensive Care Med 25:1140–1146
Sigurdsson S, Svantesson C, Larsson A, Jonson B (2000) Elastic pressure-volume curves indicate derecruitment after a single deep expiration in anaesthetised and muscle-relaxed healthy man. Acta Anaesthesiol Scand 44:980–984
De Robertis E, Liu JM, Blomquist S, Dahm PL, Thörne J, Jonson B (2001) Elastic properties of the lung and the chest wall in young and adult healthy pigs. Eur Respir J 17:703–711
Acknowledgements
The study was supported by the Swedish Research Council (02872) and by the Swedish Heart-Lung Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Bitzén, U., Drefeldt, B., Niklason, L. et al. Dynamic elastic pressure-volume loops in healthy pigs recorded with inspiratory and expiratory sinusoidal flow modulation. Intensive Care Med 30, 481–488 (2004). https://doi.org/10.1007/s00134-003-2156-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-2156-6