Abstract
In this article, the authors present the building of a new cardiac output (CO) simulator that aims to provide a reliable pre-surgical test of instruments used by clinical teams to measure the patient’s condition during pulmonary artery catheterization. This procedure applies the thermodilution method, in which the Swan-Ganz catheter (also known as pulmonary artery catheter) and the hemodynamic monitor are used to collect real-time patient data. The authors designed, built, and tested a system containing both hardware and software prototypes to simulate the human body and cardiac monitor performances. The shortcoming of current simulators available commercially in the market is related to the fact that they generate electrical signals for the input of the cardiac monitor, reproducing the variations of the temperature sensor (thermistor) of the pulmonary artery catheter, but not physically providing the thermodilution curve for the sensor. The novelty of this project compared to existing simulators is the creation of a device with superior environment testing capabilities, covering the entire system with catheter and cardiac monitor connected, physically creating temperature variations on the catheter’s sensor and at the same time reproducing the cardiac output monitor calculation in the computer. The prototype showed similar accuracy compared to existing simulators of ± 0.1 L/min approximately (considering a total of 18 trials with standard deviation of 0.05745 L/min), but with the competitive advantage of creating the thermodilution curve for the catheter’s thermistor with real temperature environment and calculating CO value in real time. The prototype was able to provide similar simulation options as the software database contains a large range of catheter models, injectate volumes, injectate temperatures, and computation constants compared to the literature from MicroSim COS®, SimSlim® SL-8, PS-2200® Series, AMPS-1®, Seculife PS300®, ProSim® 3, and ProSim® 8 simulators. With such a pre-surgical test equipment, the irregularities in the cardiac output invasive monitoring system, and more specifically the limitations in the current method of checking the accuracy of the pulmonary artery catheter’s temperature sensor and CO monitor’s calibration, could be identified and mitigated before the catheterization. Thus, it could avoid complications (e.g., malfunction and infections), reducing costs and delays in medical treatments due to non-calibrated devices which are not in proper conditions of functioning for ICU staff and healthcare teams. Beyond it, the developed simulator can be used as educational tool for cardiac catheterization, helping to train medical and clinical professionals and contributing to the design iteration of new cardiac simulation devices.

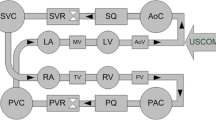
Source Clinical Gate (2015) [344]


Source Authors (2023)

Source Authors (2023)

Source The authors (2023)

Source: Liu et al. [205]

Source The authors (2023)

Source The authors (2023)

Source The authors (2023)

Source The authors (2023)

Source Authors (2023)

Source Authors (2023)

Source Authors (2023)

Source The authors (2023)

Source Authors (2023)

Source Authors (2023)

Source Authors (2023)

Source The authors (2023)

Source The authors (2023)
Similar content being viewed by others
Data Availability
Supplementary data are available upon reasonable request. Further inquiries can be directed to the corresponding author.
References
World Health Organization. Cardiovascular disease (CVDs) (2021). https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed 10 July 2023
Centers for Disease Control and Prevention. Heart Disease Facts (2023). https://www.cdc.gov/heartdisease/facts.htm. Accessed 10 July 2023
J.V. Diaz, E.D. Riviello, A. Papali et al., Global critical care: moving forward in resource-limited settings. Ann. Glob. Health 85(1), 11 (2019). https://doi.org/10.5334/aogh.2413
A.D. Shaw, M.G. Mythen, D. Shook et al., Pulmonary artery catheter use in adult patients undergoing cardiac surgery: a retrospective, cohort study. Perioper. Med. 7(24), 1–11 (2018). https://doi.org/10.1186/s13741-018-0103-x
M.R. Pinsky, J.-L. Teboul, J.-L. Vincent, Hemodynamic Monitoring (Springer, New York, 2019), p.479. https://doi.org/10.1007/978-3-319-69269-2
Fluke Biomedical. ProSim 8 Vital Signs Simulator Technical Data, p. 12 (2013). https://www.flukebiomedical.com/sites/default/files/resources/Prosim8_ENG_I_W.PDF. Accessed 10 July 2023
A. Yartsev, Measurement of cardiac output by indicator dilution. In: Deranged Physiology (2015–2020). https://derangedphysiology.com/main/cicm-primary-exam/required-reading/cardiovascular-system/Chapter%20810/measurement-cardiac-output-indicator-dilution. Accessed 10 July 2023
Edwards Lifesciences Corporation. Normal hemodynamic parameters and laboratory values. PP—US-2312 v2.0, Mar 2022, p. 4 (2022). https://educationgb.edwards.com/normal-hemodynamic-parameters-pocket-card/1167897#swan-ganz. Accessed 10 July 2023
E.E. Argueta, D. Paniagua, Thermodilution cardiac output—a concept over 250 years in the making. Cardiol. Rev. 27(3), 138–144 (2019). https://doi.org/10.1097/CRD.0000000000000223
E. Robin, M. Costecalde, G. Lebuffe et al., Clinical relevance of data from the pulmonary artery catheter. Crit. Care 10(3), 1–10 (2006). https://doi.org/10.1186/cc4830
K. Scales, E. Collie, A practical guide to using pulmonary artery catheters. Nurs. Stand. 21(43), 42–48 (2007). https://doi.org/10.7748/NS2007.07.21.43.42.C4577
I.T. Bootsma, E.C. Boerma, F. de Lange et al., The contemporary pulmonary artery catheter. Part 1: placement and waveform analysis. J. Clin. Monit. Comput. 36, 5–15 (2021). https://doi.org/10.1007/s10877-021-00662-8
Research and Markets. Global Pulmonary Artery Catheter Market Report and Forecast 2023–2031. p. 147 (2023). https://www.researchandmarkets.com/reports/5805721/global-pulmonary-artery-catheter-market-report. Accessed 10 July 2023
M. Stevens, T. Davis, S.H. Munson et al., Short and mid-term economic impact of pulmonary artery catheter use in adult cardiac surgery: a hospital and integrated health system perspective. ClinicoEconomics Outcomes Res. 13, 109–119 (2021)
Practice guidelines for pulmonary artery catheterization, An updated report by the American Society of Anesthesiologists Task Force on Pulmonary Artery Catheterization. Anesthesiology 99(4), 988–1014 (2003). https://doi.org/10.1097/00000542-200310000-00036
Edwards Lifesciences Corporation. Advanced Hemodynamic Monitoring Swan-Ganz Pulmonary Artery Catheter (2022). https://edwardsprod.blob.core.windows.net/media/Default/devices/monitoring/hemodynamic%20monitoring/swan-ganz_poster.pdf. Accessed 10 June 2023
W. Ganz, R. Donoso, H.S. Marcus et al., A new technique for measurement of cardiac output by thermodilution in man. Am. J. Cardiol. 27(4), 392–396 (1971). https://doi.org/10.1016/0002-9149(71)90436-X
W. Ganz, H.J.C. Swan, Measurement of blood flow by thermodilution. Am. J. Cardiol. 29(2), 241–246 (1972). https://doi.org/10.1016/0002-9149(72)90635-2
A. Yartsev, Thermodilution measurement of cardiac output by PA catheter. In: Deranged Physiology (2015–2022). https://derangedphysiology.com/main/cicm-primary-exam/required-reading/cardiovascular-system/Chapter%20813/thermodilution-measurement-cardiac-output-pa-catheter. Accessed 10 June 2023
V.K. Arya, W. Al-Moustadi, V. Dutta, Cardiac output monitoring—invasive and noninvasive. Curr. Opin. Crit. Care 28(3), 340–347 (2022). https://doi.org/10.1097/MCC.0000000000000937
J. Kobe, N. Mishra, V.K. Arya et al., Cardiac output monitoring: Technology and choice. Ann. Cardiac Anaesth. 22, 6–17 (2019). https://doi.org/10.4103/aca.ACA_41_18
M. Flick, A. Joosten, T.W.L. Scheeren et al., Haemodynamic monitoring and management in patients having noncardiac surgery: a survey among members of the European Society of Anaesthesiology and Intensive Care. Eur. J. Anaesthesiol. Intensive Care Med. 2, e0017 (2023). https://doi.org/10.1097/EA9.0000000000000017
T.W.L. Scheeren, M.A.E. Ramsay, New developments in hemodynamic monitoring. J. Cardiothorac. Vasc. Anesth. 33, S67–S72 (2019). https://doi.org/10.1053/j.jvca.2019.03.043
B. Saugel, J.L. Vincent, Cardiac output monitoring: how to choose the optimal method for the individual patient. Cur. Opin. Crit. Care 24(3), 165–172 (2018). https://doi.org/10.1097/MCC.0000000000000492
X. Monnet, J.-L. Teboul, Transpulmonary thermodilution: advantages and limits. Crit. Care 21, 147 (2017). https://doi.org/10.1186/s13054-017-1739-5
R.H. Thiele, K. Bartels, T.J. Gan, Cardiac output monitoring: a contemporary assessment and review. Crit. Care Med. 43(1), 177–185 (2015). https://doi.org/10.1097/CCM.0000000000000608
L. Sangkum, G.L. Liu, L. Yu et al., Minimally invasive or noninvasive cardiac output measurement: an update. J. Anesth. 30(3), 461–480 (2016). https://doi.org/10.1007/s00540-016-2154-9
J.A. Alhashemi, M. Cecconi, C.K. Hofer, Cardiac output monitoring: an integrative perspective. Crit. Care 15, 214 (2011). https://doi.org/10.1186/cc9996
D.A. Reuter, C. Huang, T. Edrich, S.K. Shernan, H.K. Eltzschig, Cardiac output monitoring using indicator-dilution techniques: basics, limits, and perspectives. Anesth. Analg. 110(3), 799–811 (2010). https://doi.org/10.1213/ANE.0b013e3181cc885a
Edwards Lifesciences Corporation. A comprehensive hemodynamic profile to guide your treatment strategy. PP--US-2029 v3.0 4 p (2020). https://edwardsprod.blob.core.windows.net/media/Br/devices/monitoring/hemodynamic%20monitoring/swan-ganz_brochure.pdf. Accessed 10 July 2023
G.A. Hernandez, A. Lemor, V. Blumer, C.A. Rueda, S. Zalawadiya, L.W. Stevenson, J. Lindenfeld, Trends in utilization and outcomes of pulmonary artery catheterization in heart failure with and without cardiogenic shock. J. Card. Fail. 25(5), 364–371 (2019). https://doi.org/10.1016/j.cardfail.2019.03.004
S. Rosenkranz, I.R. Preston, Right heart catheterisation: best practice and pitfalls in pulmonary hypertension. Eur. Respir. Rev. 24(138), 642–652 (2015). https://doi.org/10.1183/16000617.0062-2015
D.C. Evans, V.A. Doraiswamy, M.P. Prosciak et al., Complications associated with pulmonary artery catheters: a comprehensive clinical review. Scand. J. Surg. 98(4), 199–208 (2009). https://doi.org/10.1177/145749690909800402
B. Shin, T.C. McAslam, R.J. Ayella, Problems with measurement using the Swan-Ganz catheter. Anesthesiology 43(4), 474–476 (1975). https://doi.org/10.1097/00000542-197510000-00012
C.G. Elliott, G.A. Zimmerman, T.P. Clemmer, Complications of pulmonary artery catheterization in the care of critically ill: a prospective study. Chest 76(6), 647–652 (1979). https://doi.org/10.1378/chest.76.6.647
M.J. Sise, P. Hollingsworth, J.E. Brimm et al., Complications of the flow-directed pulmonary artery catheter: a prospective analysis in 219 patients. Crit. Care Med. 9(4), 315–318 (1981). https://doi.org/10.1097/00003246-198104000-00006
M.C. Bessette, L. Quintin, D.G. Whalley et al., Swan-Ganz catheter contamination: a protective sleeve for repositioning. Can. Anaesth. Soc. J. 28(1), 86–88 (1981). https://doi.org/10.1007/BF03007298
K.D. Boyd, S.J. Thomas, J. Gold et al., A prospective study of complications of pulmonary artery catheterizations in 500 consecutive patients. Chest 84(3), 245–249 (1983). https://doi.org/10.1378/chest.84.3.245
F.W. Campbell, A.J. Schwartz, Pulmonary artery catheter malfunction? Anesthesiology 60(5), 513–514 (1984). https://doi.org/10.1097/00000542-198405000-00036
S.A. Shenaq, G.P. Noon, J.L. Zamora et al., Unusual complication of Swan-Ganz catheter requiring mediastinotomy. S. Med. J. 77(10), 1339 (1984). https://doi.org/10.1097/00007611-198410000-00039
M.L. Myers, T.W. Austin, W.J. Sibbald, Pulmonary artery catheter infections: a prospective study. Ann. Surg. 201(2), 237–241 (1985)
J. Damen, D. Bolton, A prospective analysis of 1400 pulmonary artery catheterizations in patients undergoing cardiac surgery. Acta Anesthesiol. Scand. 30(5), 386–392 (1986). https://doi.org/10.1111/j.1399-6576.1986.tb02436.x
J.I. Gotchall, L. Comried, G. Bredlau et al., Evaluation of an inaccurate pulmonary artery catheter thermistor. Chest 96(4), 941–943 (1989). https://doi.org/10.1378/chest.96.4.941
M.M. Zion, J. Balkin, D. Rosenmann et al., Use of pulmonary artery catheters in patients with acute myocardial infarction. Analysis of experience in 5841 patients in the SPRINT Registry. SPRINT Study Group. Chest 98(6), 1331–1335 (1990). https://doi.org/10.1378/chest.98.6.1331
L.A. Mermel, R.D. McCormick, S.R. Springman et al., The pathogenesis and epidemiology of catheter-related infection with pulmonary artery Swan-Ganz catheters: a prospective study utilizing molecular subtyping. Am. J. Med. 91(3B), 197S-205S (1991). https://doi.org/10.1016/0002-9343(91)90369-9
V.R. Patla, C. Lorenzi, J.P. Benson et al., A faulty pulmonary artery flotation catheter. Anaesthesia 48, 828–828 (1993). https://doi.org/10.1111/j.1365-2044.1993.tb07618.x
T. Nishikawa, S. Dohi, Errors in the measurement of cardiac output by thermodilution. Can. J. Anaesthes. 40(2), 142–153 (1993). https://doi.org/10.1007/BF03011312.pdf
L.A. Mermel, D. Maki, Infectious complications of Swan-Ganz pulmonary artery catheters. Pathogenesis, epidemiology, prevention, and management. Am. J. Respir. Crit. Care Med. 149(4 Pt 1), 1020–1036 (1994). https://doi.org/10.1164/ajrccm.149.4.8143037
S. Kiyama, A dampened waveform due to a faulty pulmonar artery catheter. Anaesthesia 50(6), 566 (1995). https://doi.org/10.1111/j.1365-2044.1995.tb06061.x
T.J. Kearney, M.M. Shabot, Pulmonary artery rupture associated with the Swan-Ganz catheter. Chest 108(5), 1349–1352 (1995). https://doi.org/10.1378/chest.108.5.1349
A.F. Connors Jr., T. Speroff, N.V. Dawson et al., The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA 276(11), 889–897 (1996). https://doi.org/10.1001/jama.276.11.889
S. Colbert, D.M. O’Hanlon, D.S. Quill et al., Swan-Ganz catheter—all in a knot. Eur. J. Anaesthesiol. 14(5), 518–520 (1997). https://doi.org/10.1046/j.1365-2346.1997.00201.x
J. Rello, P. Jubert, M.E. Esandi et al., Specific problems of arterial, swan-ganz, and hemodialysis catheters. Nutrition 13(4), 36S-41S (1997). https://doi.org/10.1016/s0899-9007(97)00221-9
R. Kodavatiganti, C.J. Hearn, S.R. Insler, Bleeding from a pulmonary artery catheter temperature connection port. J. Cardiothorac. Vasc. Anesth. 13(1), 75–77 (1999). https://doi.org/10.1016/S1053-0770(99)90179-9
I.C. Baldwin, M. Heland, Incidence of cardiac dysrhythmias in patients during pulmonary artery catheter removal after cardiac surgery. Heart Lung 29(3), 155–160 (2000). https://doi.org/10.1067/mhl.2000.106937
R. Ivanov, J. Allen, J.E. Calvin, The incidence of major morbidity in critically ill patients managed with pulmonary artery catheters: a meta-analysis. Crit. Care Med. 28(3), 615–619 (2000). https://doi.org/10.1097/00003246-200003000-00002
G.R. Manecke, J.C. Brown, A.A. Landau et al., An unusual case of pulmonary artery catheter malfunction. Anesth. Analg. 95(2), 302–304 (2002). https://doi.org/10.1097/00000539-200208000-00008
M.C. Lopes, R. de Cleva, B. Zilberstein et al., Pulmonary artery catheter complications: report on a case of a knot accident and literature review. Rev. Hosp. Clín. Fac. Med. 59(2), 77–85 (2004). https://doi.org/10.1590/S0041-87812004000200006
S. Harvey, D.A. Harrison, M. Singer et al., Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. The Lancet 366(9484), 472–477 (2005). https://doi.org/10.1016/S0140-6736(05)67061-4
M. Hadian, M.R. Pinsky, Evidence-based review of the use of the pulmonary artery catheter: impact data and complications. Crit. Care 10(Suppl 3), 1–11 (2006). https://doi.org/10.1186/cc4834
S.E. Harvey, C.A. Welch, D.A. Harrison et al., Post hoc insights from PAC-Man—the U.K. pulmonary artery catheter trial. Crit. Care Med. 36(6), 1714–1721 (2008). https://doi.org/10.1097/CCM.0b013e318174315d
A. Katsikis, G. Karavolias, V. Voudris, Transfemoral percutaneous removal of a knotted Swan-Ganz catheter. Catheter. Cardiov. Interv. 74(5), 802–804 (2009). https://doi.org/10.1002/ccd.22201
W.-H. Chan, C.-H. Hsu, C.-C. Lu et al., Early recognition of an entrapped pulmonary artery catheter by blood leaking into the syringe and thermistor connector during cardiac surgery. Acta Anaesthesiol. Taiwan. 50(1), 38–40 (2012). https://doi.org/10.1016/j.aat.2012.03.003
M. Ishaq, N. Alexander, D.H.T. Scott, Successful retrieval of a knotted pulmonary artery catheter trapped in a tricuspid valve apparatus. Saudi J. Anaesth. 7(2), 191–193 (2013). https://doi.org/10.4103/1658-354X.114048
S.-K. Park, N.-S. Gil, H.-G. Ryu, A misplaced and entrapped pulmonary artery catheter. Korean J. Anesthesiol. 64(4), 380–381 (2013)
P.E. Marik, Obituary: pulmonary artery catheter 1970 to 2013. Ann. Intensive Care 3, 1–6 (2013). https://doi.org/10.1186/2110-5820-3-38
U.K. Gidwani, B. Mohanty, K. Chatterjee, The pulmonary artery catheter: a critical reappraisal. Cardiol. Clin. 31(4), 545–565 (2013). https://doi.org/10.1016/j.ccl.2013.07.008
H.J. Lee, N. Kim, H. Lee et al., Persistent left superior vena cava detected incidentally after pulmonary artery catheterization. KJCCM 30(1), 22–26 (2015). https://doi.org/10.4266/kjccm.2015.30.1.22
E.Y. Brovman, R.A. Gabriel, R.P. Dutton et al., Pulmonary artery catheter use during cardiac surgery in the United States, 2010 to 2014. J. Cardiothorac. Vasc. Anesth. 30(3), 579–584 (2016). https://doi.org/10.1053/j.jvca.2015.11.012
K. Slicker, W.G. Lane, O.O. Oyetano et al., Daily cardiac catheterization procedural volume and complications at an academic medical center. Cardiovasc. Diagn. Ther. 6, 446–452 (2016). https://doi.org/10.21037/cdt.2016.05.02
I.T. Bootsma, E.C. Boerma, T.W.L. Scheeren et al., The contemporary pulmonary artery catheter. Part 2: measurements, limitations, and clinical applications. J. Clin. Monit. Comput. 36, 17–31 (2021). https://doi.org/10.1007/s10877-021-00673-5
N. Takai, A. Kambara, J. Iemura et al., Damage to pulmonary artery catheter in cardiac surgery causing inadequate pulmonary artery pressure waveform. Cardiov. Anesth. 25(1), 75–78 (2021)
A. Yartsev, Causes of inaccurate thermodilution cardiac output measurements. In: Deranged Physiology (2015–2023). https://derangedphysiology.com/main/required-reading/equipment-and-procedures/Chapter%20236/causes-inaccurate-thermodilution-cardiac-output-measurements. Accessed 14 July 2023
Netech Corporation. MicroSim COS—Cardiac Output Simulator Operating Manual, p. 16 (2011). https://www.netechbiomedical.com/image/catalog/pdf/microsim_cos_1111_user_manual.pdf. Accessed 10 July 2023
Pronk Technologies. Specifications SimSlim Model SL-8, p. 2 (2020). https://www.pronktech.com/wp-content/uploads/2020/07/SimSlim-SL-8-Specifications-2020-04-14.pdf. Accessed 11 July 2023
Pronk Technologies. SimSlim Patient Simulator Operator’s Manual (2017). https://www.pronktech.com/wp-content/uploads/2015/02/501-0607-SIMSLIMMANUAL-REV-L.pdf. Accessed 9 July 2023
BC Group International. PS2200 Series Specification Sheet—Multi-Parameter Simulators. Brochure, p. 2 p (2020). https://www.bcgroupstore.com/Assets/PDF/DataSheets/BCBiomedicalPS2200SeriesSpecSheet.pdf. Accessed 10 July 2023
BC Biomedical. Multi-Parameter Patient Simulator PS-2200 Series User Manual, p. 78 (2020). https://www.bcgroupstore.com/Assets/PDF/manuals/PS-2200_User_Manual.pdf. Accessed 10 July 2023
Datrend Systems Incorporated. AMPS-1 Advanced Modular Patient Simulator, p. 2 (2016). https://www.datrend.com/download.php?file=AMPS-1_Spec_Oct_2016.pdf. Accessed 10 July 2023
Datrend Systems Incorporated. AMPS-1 Advanced Modular Patient Simulator Operating Manual, p. 100 (2021). https://www.datrend.com/download.php?file=MN-053f-6100-453-AMPS-1-Operators-Manual.pdf. Accessed 11 July 2023
Gossen Metrawatt. SECULIFE PS300 Multi-Patient Simulator, p. 62 (2013). https://www.gmc-instruments.de/media/doku/pm/seculife-ps300/seculife-ps300-ba_gb.pdf. Accessed 10 July 2023
Fluke Biomedical. ProSim 2/3 User’s Manual. Rev. 1, p. 60 (2013). https://www.flukebiomedical.com/sites/default/files/resources/prosim3_umeng0100.pdf. Accessed 10 July 2023
Fluke Biomedical. ProSim 8 Vital Sign Simulator. Users Manual (2011). https://www.flukebiomedical.com/sites/default/files/resources/prosim8_umeng0300.pdf. Accessed 10 July 2023
Society for Science. Intel International Science and Engineering Fair 2012 Program. 148 p. Simdeb II project on p. 61 (2012). https://sspcdn.blob.core.windows.net/files/Documents/SEP/ISEF/2012/Program-Book.pdf. Accessed 10 July 2023
C.F.T. Abreu, Simdeb II—Simulador de débito cardíaco (abstract in portuguese, p. 179). In: Mostratec. Resumos da Mostratec v.3, 2011, p. 309 (2011). https://www.mostratec.com.br/wp-content/uploads/2020/08/resumo_da_mostratec_v._3_2011.pdf. Accessed 11 July 2023
C.F.T. Abreu, D. Johann, Simdeb—Simulador de débito cardíaco (abstract in portuguese, p. 217). In: Mostratec. Resumos da Mostratec v.2, 2010, p. 302 (2010). https://www.mostratec.com.br/wp-content/uploads/2020/08/resumos_da_mostratec_v._2_2010.pdf. Accessed 11 July 2023
W.F. Hamilton, J.W. Moore, J.M. Kinsman et al., Simultaneous determination of the pulmonary and systemic circulation times in man and of a figure related to the cardiac output. Am. J. Physiol. 84(2), 338–344 (1928). https://doi.org/10.1152/ajplegacy.1928.84.2.338
G. Fegler, Measurement of cardiac output in anaesthetized animals by a thermodilution method. Q. J. Exp. Physiol. Cogn. Med. Sci. 39(3), 153–164 (1954). https://doi.org/10.1113/expphysiol.1954.sp001067
T. Cooper, E. Braunwald, G.C. Riggles et al., Thermal dilution curves in the study of circulatory shunts: instrumentation and clinical applications. Am. J. Cardiol. 6(6), 1065–1069 (1960). https://doi.org/10.1016/0002-9149(60)90362-3
E. Evonuk, C.J. Imig, W. Greenfield, J.W. Eckstein, Cardiac output measured by thermal dilution of room temperature injectate. J. Appl. Physiol. 16, 271–275 (1961). https://doi.org/10.1152/jappl.1961.16.2.271
K.L. Zierler, Theoretical basis of indicator-dilution methods for measuring flow and volume. Circ. Res. 10, 393 (1962). https://doi.org/10.1161/01.RES.10.3.393
K. Pávek, D. Boska, F.V. Selecký, Measurement of cardiac output by thermodilution with constant rate injection of indicator. Circ. Res. 15, 311–319 (1964). https://doi.org/10.1161/01.res.15.4.311
G.W. James, M.H. Paul, H.U. Wessel, Thermal dilution: instrumentation with thermistors. J. Appl. Physiol. 20(3), 547–552 (1965). https://doi.org/10.1152/jappl.1965.20.3.547
E. Rapaport, Usefulness and limitations of thermal washout technics in ventricular volume measurements. Am. J. Cardiol. 18, 226–234 (1966). https://doi.org/10.1016/0002-9149(66)90035-X
C.R. Salgado, P.M. Galletti, In vitro evaluation of the thermodilution technique for the measurement of ventricular stroke volume and end-diastolic volume. Cardiologia 49(2), 65–78 (1966). https://doi.org/10.1159/000168893
G.A. Cropp, Measurements of variable ventricular output by thermodilution: model experiments. J. Appl. Physiol. 21(5), 1624–1632 (1966). https://doi.org/10.1152/jappl.1966.21.5.1624
J.C.P. Williams, T.P.B. O’Donovan, E.H. Wood, A method for the calculation of areas under indicator-dilution curves. J. Appl. Physiol. 21(2), 695–699 (1966). https://doi.org/10.1152/jappl.1966.21.2.695
H.J. Swan, W. Ganz, J. Forrester et al., Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N. Engl. J. Med. 283(9), 447–451 (1970). https://doi.org/10.1056/NEJM197008272830902
B. Olsson, J. Pool, P. Vandermoten et al., Validity and reproducibility of determination of cardiac output by thermodilution in man. Cardiology 55(3), 136–48 (1970). https://doi.org/10.1159/000169277
E. Pavek, K. Pavek, D. Boska, Mixing and observation errors in indicator-dilution studies. J. Appl. Physiol. 28(6), 733–740 (1970). https://doi.org/10.1152/jappl.1970.28.6.733
M.E. Sanmarco, C.M. Philips, L.A. Marquez et al., Measurement of cardiac output by thermal dilution. Am. J. Cardiol. 28(1), 54–58 (1971). https://doi.org/10.1016/0002-9149(71)90034-8
H.U. Wessel, M.H. Paul, G.W. James et al., Limitations of thermal dilution curves for cardiac output determinations. J. Appl. Physiol. 30(5), 643–652 (1971). https://doi.org/10.1152/jappl.1971.30.5.643
K.-E. Arfors, P. Malmberg, K. Pavek, Conservation of thermal indicator in lung circulation. Cardiovasc. Res. 5(4), 530–534 (1971). https://doi.org/10.1093/cvr/5.4.530
R.F. Leighton, J. Czekajewski, Use of a new cardiac output computer for human hemodynamics studies. J. Appl. 30(6), 914–916 (1971). https://doi.org/10.1152/jappl.1971.30.6.914
J.S. Forrester, W. Ganz, G. Diamond et al., Thermodilution cardiac output determination with single flow-directed catheter. Am. Heart J. 83(3), 306–311 (1972). https://doi.org/10.1016/0002-8703(72)90429-2
E.M. Wilson, A.J. Ranieri Jr., O.L. Updike et al., An evaluation of thermal dilution for obtaining serial measurements of cardiac output. Med. Biol. Eng. 10, 179–191 (1972). https://doi.org/10.1007/BF02474108
R.J. Ellis, J. Gold, J.R. Rees et al., Computerized monitoring of cardiac output by thermal dilution. JAMA 220(4), 507–511 (1972). https://doi.org/10.1001/jama.1972.03200040029006
K. Tamura, Y. Arai, H. Murooka, The rapid-estimation of the area under the thermodilution curve. Jpn. Heart J. 14(4), 306–313 (1973)
H. Meisner, S. Haql, W. Heimish et al., Evaluation of the thermodilution method for measurement of cardiac output after open-heart surgery. Ann. Thorac. Surg. 18(5), 504–515 (1974). https://doi.org/10.1016/s0003-4975(10)64393-7
M. Andreen, Computerized measurement of cardiac output by thermodilution: methodological aspects. Acta Anaesthesiol. Scand. 18(4), 297–305 (1974). https://doi.org/10.1111/j.1399-6576.1974.tb01183.x
R.L. Berger, R.D. Weisel, L. Vito et al., Cardiac output measurement by thermodilution during cardiac operations. Ann. Thorac. Surg. 21(1), 43–47 (1976). https://doi.org/10.1016/s0003-4975(10)64886-2
J. Beyer, J.J. Lamberti, R.L. Replogle, Validity of thermodilution cardiac output determination: experimental studies with and without pulmonary insufficiency. J. Surg. Res. 21(5), 313–317 (1976). https://doi.org/10.1016/0022-4804(76)90043-3
L.H. Snoeckx, J.L. Verheyen, A. Van de Water et al., On-line computation of cardiac output with the thermodilution method, using a digital minicomputer. Cardiovasc. Res. 10(5), 556–564 (1976). https://doi.org/10.1093/cvr/10.5.556
F.H. Kohanna, J.N. Cunningham Jr., Monitoring of cardiac output by thermodilution after open-heart surgery. J. Thorac. Cardiovasc. Surg. 73(3), 451–457 (1977)
J. Davis, Flow bench for the evaluation of thermal dilution cardiac output computers. J. Extra-Corp Tech. 9(4), 187–195 (1977)
G.M. White, C.N. Murthy, A computer program for the on-line computation of cardiac output from thermodilution curves. Comput. Prog. Biomed. 7(1), 37–40 (1977). https://doi.org/10.1016/0010-468x(77)90034-4
C.T. Dizon, W.A. Gezari, P.G. Barash et al., Hand held thermodilution cardiac output injector. Crit. Care Med. 5(4), 210–212 (1977). https://doi.org/10.1097/00003246-197707000-00011
B.L. Hoel, Some aspects of the clinical use of thermodilution in measuring cardiac output. With particular reference to the Swan-Ganz thermodilution catheters. Scand. J. Clin. Lab Invest. 38(4), 383–388 (1978). https://doi.org/10.3109/00365517809108438
A.P. Fischer, A.M. Benis, R.A. Jurado, E. Seely, P. Teirstein, R.S. Litwak, Analysis of errors in measurement of cardiac output by simultaneous dye and thermal dilution in cardiothoracic surgical patients. Cardiovasc. Res. 12(3), 190–199 (1978). https://doi.org/10.1093/cvr/12.3.190
D.S. Moodie, R.H. Feldt, M.P. Kaye, D.A. Strelow, L.J. van der Hagen, Measurement of cardiac output by thermodilution: development of accurate measurements at flows applicable to the pediatric patient. J. Surg. Res. 25(4), 305–311 (1978). https://doi.org/10.1016/0022-4804(78)90123-3
M. Wong, A. Skulsky, E. Moon, Loss of indicator in the thermodilution technique. Cathet. Cardiovasc. Diagn. 4(1), 103–109 (1978). https://doi.org/10.1002/ccd.1810040115
J.M. Levett, R.L. Replogle, Thermodilution cardiac output: a critical analysis and review of the literature. J. Surg. Res. 27(6), 392–404 (1979). https://doi.org/10.1016/0022-4804(79)90159-8
J.J. Stawicki, F.D. Holford, E.L. Michelson et al., Multiple cardiac output measurements in man. Evaluation of a new closed-system thermodilution method. Chest 76(2), 193–197 (1979). https://doi.org/10.1378/chest.76.2.193
M.E. Kim, Y.C. Lin, Determination of catheter wall heat transfer in cardiac output measurement by thermodilution. Clin. Exp. Pharmacol. Physiol. 7(4), 383–389 (1980). https://doi.org/10.1111/j.1440-1681.1980.tb00086.x
W.B. Runciman, A.H. Ilsey, J.G. Roberts, Thermodilution cardiac output—a systematic error. Anaesth. Intensive Care 9(2), 135–139 (1981). https://doi.org/10.1177/0310057X8100900206
J.R. Plachetka, D.F. Larson, N.W. Salomon et al., Comparison of two closed systems for thermodilution cardiac outputs. Crit. Care Med. 9(6), 487–489 (1981). https://doi.org/10.1097/00003246-198106000-00011
W.B. Runciman, A.H. Isley, J.G. Roberts, An evaluation of thermodilution cardiac output measurement using the Swan-Ganz catheter. Anaesth. Intensive Care 9(3), 208–220 (1981). https://doi.org/10.1177/0310057X8100900302
T.V. Bilfinger, C.-Y. Lin, C.D. Anagnostopoulos, In vitro determination of accuracy of cardiac output measurements by thermal dilution. J. Surg. Res. 33, 409–414 (1982). https://doi.org/10.1016/0022-4804(82)90056-7
C.W. Stetz, R.G. Miller, G.E. Kelly et al., Reliability of the thermodilution method in the determination of cardiac output in clinical practice. Am. Rev. Respir. Dis. 126(6), 1001–1004 (1982). https://doi.org/10.1164/arrd.1982.126.6.1001
U. Elkayam, R. Berkley, S. Azen et al., Cardiac output by thermodilution technique: effect of injectate’s volume and temperature on accuracy and reproducibility in the critically Ill patient. Chest 84(4), 418–422 (1983). https://doi.org/10.1378/chest.84.4.418
F.G. Shellock, M.S. Riedinger, T.M. Bateman et al., Thermodilution cardiac output determination in hypothermic postcardiac surgery patients: room vs ice temperature injectate. Crit. Care Med. 11(8), 668–670 (1983). https://doi.org/10.1097/00003246-198308000-00018
H.R. Kay, M. Afshari, P. Barash et al., Measurement of ejection fraction by thermal dilution techniques. J. Surg. Res. 34(4), 337–346 (1983). https://doi.org/10.1016/0022-4804(83)90081-1
J.H. Philip, M.C. Long, M.D. Quinn et al., Continuous thermal measurement of cardiac output. IEEE Trans. Biomed. Eng. 31(5), 393–400 (1984). https://doi.org/10.1109/TBME.1984.325278
G.F. Maruschak, J.F. Schauble, Limitations of thermodilution ejection fraction: degradation of frequency response by fast-response thermistors. Crit. Care Med. 13(8), 679–682 (1985). https://doi.org/10.1097/00003246-198508000-00015
R.C. Wetzel, T.W. Latson, Major errors in thermodilution cardiac output measurements during rapid volume infusion. Anesthesiology 62(5), 684–687 (1985). https://doi.org/10.1097/00000542-198505000-00035
R.G. Pearl, M.H. Rosenthal, L. Nielson et al., Effect of injectate volume and temperature on thermodilution cardiac output determination. Anesthesiology 64(6), 798–801 (1986). https://doi.org/10.1097/00000542-198606000-00021
P.J.R. Jebson, W.S. Karkow, Pulsatile flow simulator for comparison of cardiac output measurements by electromagnetic flow meter and thermodilution. J. Clin. Monit. 2(1), 6–14 (1986). https://doi.org/10.1007/BF01619172
J.D. Mackenzie, N.E. Haites, J.M. Rawles, Method of assessing the reproducibility of blood flow measurement: factors influencing the performance of thermodilution cardiac output computers. Br. Heart J. 55, 14–24 (1986). https://doi.org/10.1136/hrt.55.1.14
S. Nadeau, W.H. Noble, Limitations of cardiac output measurements by thermodilution. Can. Anaesth. Soc. J. 33(6), 780–784 (1986). https://doi.org/10.1007/BF03027130.pdf
S.L. Norris, E.G. King, M. Grace et al., Thermodilution cardiac output—an in vitro model of low flow states. Crit. Care Med. 14(1), 57–59 (1986). https://doi.org/10.1097/00003246-198601000-00013
R. Long, L. Wood, I. Sznadjer, An in vitro calibration of the thermodilution method of cardiac output determination. J. Extra-Corporeal Technol. 9(2), 7 (1987)
J.F. Dhainaut, F. Brunet, J.F. Monsallier et al., Bedside evaluation of right ventricular performance using a rapid computerized thermodilution method. Crit. Care Med. 15(2), 148–152 (1987). https://doi.org/10.1097/00003246-198702000-00014
S.A. Conrad, M. Jones, P. Unkel. Thermodilution cardiac output and ejection fraction: a mathematical analysis. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New Orleans, LA, USA, pp. 1855–1856, v. 4 (1988). https://doi.org/10.1109/IEMBS.1988.95123
H. Maruta, Y. Usuda, Y. Okutsu et al., A new closed-system using partially frozen injectate for thermodilution cardiac output determinations. J. Anesth. 3(1), 35–39 (1989). https://doi.org/10.1007/s0054090030035
S.S. Saliterman, A computerized simulator for critical-care training: New Technology for Medical Education. Mayo Clinic Proc. 65(7), 968–978 (1990). https://doi.org/10.1016/s0025-6196(12)65158-1
F.G. Spinale, J.L. Zellner, R. Mukherjee et al., Thermodilution right ventricular ejection fraction. Catheter positioning effects. Chest 98(5), 1259–1265 (1990). https://doi.org/10.1378/chest.98.5.1259
R. Mukherjee, F.G. Spinale, A.F. von Recum et al., In vitro validation of a right ventricular thermodilution ejection fraction system. Ann. Biomed. Eng. 19, 165–177 (1991). https://doi.org/10.1007/BF02368467
S.E. Ferris, M. Konno, In vitro validation of a thermodilution right ventricular ejection fraction method. J. Clin. Monit. 1992(8), 74–80 (1992). https://doi.org/10.1007/BF01618092
K.A. Jarvis, M.J. Woliner, E.P. Steffey, Accuracy of the thermodilution method in estimating high flow—an in vitro study. J. Vet. Anaesth. 19(1), 41–45 (1992). https://doi.org/10.1111/j.1467-2995.1992.tb00084.x
M.L. Dollar, M.L. Yelderman, M.D. Quinn et al., Evaluation of a continuous thermodilution cardiac output catheter. ASAIO J. 38(3), M351–M356 (1992). https://doi.org/10.1097/00002480-199207000-00053
J.P. Mitchell, D. Schuller, F.S. Calandrino, D.P. Schuster, Improved outcome based on fluid management in critically Ill patients requiring pulmonary artery catheterization. Am. Rev. Respir. Dis. 145(5), 990–998 (1992). https://doi.org/10.1164/ajrccm/145.5.990
L.E. Renner, M.J. Morton, G.Y. Sakuma, Indicator amount, temperature, and intrinsic cardiac output affect thermodilution cardiac output accuracy and reproducibility. Crit. Care Med. 21(4), 586–597 (1993). https://doi.org/10.1097/00003246-199304000-00021
T. Segawa, M. Arakawa, K. Kambara et al., Correction for apparent prolongation of mean transit time resulting from response time in a thermodilution system. IEEE Trans. Biom. Eng. 40, 1–7 (1993). https://doi.org/10.1109/10.204765
R.D. Zielstorff, et al., Providing clinicians with problem-based access to knowledge: troubleshooting pulmonary artery catheter waveforms. Proc Annu Symp Comput Appl Med Care, pp. 351–355 (1993). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2248530/. Accessed 10 July 2023
S. Cockroft, P.S. Withington, The measurement of right ventricular ejection fraction by thermodilution. A comparison of values obtained using differing injectate ports. Anaesthesia 48(4), 312–314 (1993). https://doi.org/10.1111/j.1365-2044.1993.tb06950.x
J.E. Williams, S.E. Pfau, L.I. Deckelbaum, Effect of injectate temperature and thermistor position on reproducibility of thermodilution cardiac output determinations. Chest 106(3), 895–898 (1994). https://doi.org/10.1378/chest.106.3.895
J. Hori, Y. Saitoh, T. Kiryu, Improvement of the time-domain response of a thermodilution sensor by the natural observation system. IEICE Trans. Fundam. E 77(5), 784–791 (1994)
T. Mihaljevic, L.K. von Segesser, M. Tönz et al., Continuous thermodilution measurement of cardiac output: in-vitro and in-vivo evaluation. Thorac. Cardiovasc. Surg. 42(1), 32–35 (1994). https://doi.org/10.1055/s-2007-1016451
A. Rubini, D. Del Monte, V. Catena et al., Cardiac output measurement by the thermodilution method: an in vitro test of accuracy of three commercially available automatic cardiac output computers. Intensive Care Med. 21(2), 154–158 (1995). https://doi.org/10.1007/BF01726539
O. Barnea, Hemo/thermodynamic model for analysis of thermodilution. Proc of the 19th annual IEEE/EMBS conference. Oct-Nov 1997, p. 4. (1997). https://doi.org/10.1109/IEMBS.1997.758796
N. Gefen, O. Barnea, A. Abramovich, et al., Experimental assessment of error sources in thermodilution measurements of cardiac output and ejection fraction. p. 796. In: Proceeding of the First Joint BMES/EMBS Conference, Oct 13–16, Atlanta, USA, 1999. (1999). https://doi.org/10.1109/IEMBS.1999.803951
K.G. Lehmann, M.S. Platt, Improved accuracy and precision of thermodilution cardiac output measurement using a dual thermistor catheter system. JACC 33(3), 9 (1999)
I. dos Santos, A.F. da Rocha, F.A.O. Nascimento et al., Measurement of ejection fraction with standard thermodilution catheters. Med. Eng. Phys. 24(5), 325–335 (2002). https://doi.org/10.1016/S1350-4533(02)00026-7
C.R. Humphrey, J.L. Cezeaux, S. Schreiner, The design and fabrication of a closed loop steady flow system for the study of thermodilution. Proceedings of the IEEE 28th Annual Northeast Bioengineering Conference (IEEE Cat. No.02CH37342) (2002). https://doi.org/10.1109/NEBC.2002.999483
P.G. Berthelsen, N. Eldrup, L.B. Nilsson et al., Thermodilution cardiac output. Cold vs room temperature injectate and the importance of measuring the injectate temperature in the right atrium. Acta Anaesthesiol. Scand. 46(9), 1103–1110 (2002). https://doi.org/10.1034/j.1399-6576.2002.460908.x
M.P. Eason, M.S. Goodrow, J.E. Gillespie, A device to stimulate central venous cannulation in the human patient simulator. Anesthesia 99(1246), 10–11 (2003). https://doi.org/10.1097/00000542-200311000-00050
L.B. Nilsson, J.C. Nilsson, L.T. Skovgaard, P.G. Berthelsen, Thermodilution cardiac output—are three injections enough? Acta Anaesthesiol. Scand. 48(10), 1322–1327 (2004). https://doi.org/10.1111/j.1399-6576.2004.00514.x
M.P. Eason, M.D. Linville, C. Stanton, A system to stimulate arterial blood flow for cannulation in the human patient simulator. Anesthesiology 103(2), 443 (2005). https://doi.org/10.1097/00000542-200508000-00031
A. Liu, Y. Bhasin, M. Fiorill, et al., The Design and Implementation of a Pulmonary Artery Catheterization Simulator. IOS Press, p. 5 (2005). https://www.simcen.org/pdf/liu%20mmvr2006.pdf. Accessed 10 July 2023
A.F. da Rocha, I. dos Santos, F.A.O. Nascimento et al., Effects of the time response of the temperature sensor on thermodilution measurements. Physiol. Meas. 26, 885–901 (2005). https://doi.org/10.1088/0967-3334/26/6/001
M.D.B. de Melo, Algoritmo para Recuperação de Sinais de Temperatura de Cateteres de Artéria Pulmonar. Univ Brasília, Depart Eng Elét, p. 115 (2007). https://repositorio.unb.br/bitstream/10482/2858/1/2007_MaxwellDiogenesBandeiradeMelo.PDF. Accessed 10 July 2023
M. dos Santos, Simulador para Testes de Curvas de Termodiluição em Monitores de Débito Cardíaco. M.S. thesis, Dept Clin Eng UFCSPA, Porto Alegre, RS, Brazil. (2009)
Y.-H. Jeong, Y.-K. Kim, Using Swan-Ganz Catheter in Cardiopulmonary Patients with More Accurate Cardiac Output Measurement Module Development. Proceedings KIICSC, pp. 473–476 (2010). https://koreascience.kr/article/CFKO201014258944026.pdf. Accessed 17 July 2023.
L.A. Critchley, A. Lee, A.M.-H. Ho, A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth. Analg. 111(5), 1180–1192 (2010). https://doi.org/10.1213/ANE.0b013e3181f08a5b
X.-X. Yang, L.A. Critchley, G.M. Joynt, Determination of the precision error of the pulmonary artery thermodilution catheter using an in vitro continuous flow test rig. Anesth. Analg. 112(1), 70–77 (2011). https://doi.org/10.1213/ANE.0b013e3181ff475e
M. Ӧzbek, H.F. Ӧzel, N. Ekerbiçer et al., A physical model of the thermodilution method: influences of the variations of experimental setup on the accuracy of flow rate estimation. Biomed. Tech. 56, 59–64 (2011). https://doi.org/10.1515/BMT.2010.058
M. Gawlikowski, T. Pustelny, Physical model of the pulmonary circulation designed for investigation on cardiac output measurement by means of the thermodilution method. Acta Physica Polonica 120(4), 798–802 (2011)
M. Gawlikowski, T. Pustelny, B. Przywara-Chowaniec et al., Theoretical and model analysis of the unreability of cardiac output measurement by means of the thermodilution method. Bull. Pol. Acad. Sci. 59(4), 435–439 (2011). https://doi.org/10.2478/v10175-011-0054-6
M. Gawlikowski, T. Pustelny, Investigations concerning the application of the cross-correlation method in cardiac output measurements. BioMed Eng. Online 11, 1–9 (2012). https://doi.org/10.1186/1475-925X-11-24
B.-S. Lim, S.-H. Han, Y.-K. Kim, The Study of continuous cardiac output measurement module development of the cardiopulmonary function patient of using the Swan-Ganz Catheter. KIICE 17(4), 959–964 (2013)
M. Ložek, B. Nedvedova, J. Havlík, Mechanical model of a cardiovascular system: Determination of cardiac output by thermodilution method. International Conference on Applied Electronics, University of West Bohemia, pp. 177–179. (2013)
B. Voss, Thermodilution Cardiac Output Computer Simulator, p. 12 (2014). http://www.frankshospitalworkshop.com/electronics/diy-cardiac_output_simulator.html. Accessed 10 July 2023
J. Ye et al., Development of cardiac output monitoring system based on the thermodilution method. Chin. J. Med. Devices 38(5), 337–340 (2014)
M. Ložek, N. Havlíčková, J. Havlík, Adaptive mechanical model of cardiovascular system regulatory processes (2015). http://bmeg.fel.cvut.cz/wp-content/uploads/2015/03/Lozek-Adaptive_mechanical_model_of_Cardiovascular_system.pdf. Accessed 10 July 2023
I. Kirkeby-Garstad, H. Trønnes, R. Stenseth et al., The precision of pulmonary artery catheter bolus thermodilution cardiac output measurement varies with the clinical situation. J. Cardiothorac. Vasc. Anesth. 29(4), 881–888 (2015). https://doi.org/10.1053/j.jvca.2014.12.016
S.C. McKenzie, K. Dunster, W. Chan et al., Reliability of thermodilution derived cardiac output with different operator characteristics. J. Clin. Monit. Comput. (2017). https://doi.org/10.1007/s10877-017-0010-6
M.D.B. de Melo, G.M. Botelho, V.F. Moreira et al., Characterization of the temporal response of the temperature sensor of Swan-Ganz catheter. IJDR 11(3), 45076–45086 (2021)
A. Johnson, G. Cupp, N. Armour et al., An inexpensive cardiovascular flow simulator for cardiac catheterization procedure using a pulmonary artery catheter. Front Med Technol. 3, 764007 (2021). https://doi.org/10.3389/fmedt.2021.764007/full
D. Barvík, J. Kubíček, N. Malinova, et al, Analysis and Measurement of Cardiac Output Based on Pulmonary Artery Thermodilution in Laboratory Conditions. in 8th European Medical and Biological Engineering Conference. EMBEC 2020, IFMBE Proceedings, vol. 80, ed. T. Jarm, A. Cvetkoska, S. Mahnič-Kalamiza, D. Miklavcic (Springer, Cham, 2021), pp. 73–83. https://doi.org/10.1007/978-3-030-64610-3_10
Q. Guo, X. Wu, Measuring cardiac output through thermodilution based on machine learning. J. Mech. Med. Biol. 21(5), 2140003 (2021). https://doi.org/10.1142/S0219519421400030
L. Fresiello, K. Muthiah, K. Goetschalckx et al., Initial clinical validation of a hybrid in silico—in vitro cardiorespiratory simulator for comprehensive testing of mechanical circulatory support systems. Front. Physiol. 13, 967449 (2022). https://doi.org/10.3389/fphys.2022.967449/full
E.J. Stanger, D.C. Berger, H. Jenni et al., Behaviour and stability of thermodilution signals in a closed extracorporeal circuit: a bench study. J. Clin. Monit. Comput. 37, 1095–1102 (2023)
H.J. Goldsmid, The Physics of Thermoelectric Energy Conversion. Morgan & Claypool Publishers, p. 104 (2017). https://iopscience.iop.org/book/mono/978-1-6817-4641-8.pdf. Accessed 15 July 2023
J. Smoot, Important Factors for Improved Peltier Module Reliability. In: Digi-Key Electronics (2020). https://www.digikey.com.br/pt/articles/important-factors-for-improved-peltier-module-reliability. Accessed 2 July 2023
R. Singh, Advances in the applications of thermoelectric materials, in Thermoelectricity and Advanced Thermoelectric Materials. ed. by R. Kumar, R. Singh (Elsevier, Amsterdam, 2021), pp.312–336. https://doi.org/10.1016/C2019-0-01167-3
S.H. Zaferani, M.W. Sams, R. Ghomashchi et al., Thermoelectric coolers as thermal management systems for medical applications: design, optimization, and advancement. Elsevier Nano Energy 90, 106572 (2021). https://doi.org/10.1016/j.nanoen.2021.106572
B. Hu, X.-L. Shi, J. Zou et al., Thermoelectrics for medical applications: progress, challenges, and perspectives. CEJ 437(2), 135268 (2022). https://doi.org/10.1016/j.cej.2022.135268
H. Andersson, V. Mattsson, A. Senek, Implementation of PID control using Arduino microcontrollers for glucose measurements and micro incubator applications. Uppsala Universitet, p. 22 (2015). https://www.diva-portal.org/smash/get/diva2:822215/FULLTEXT01.pdf. Accessed 28 June 2023
K.T. Zar, N.P. Aung, N. Htway, Microcontroller control thermoelectric heating and cooling system using TEC1-12706. IJTSRD 4(4), 1706–1710 (2020)
M.-W. Tian, F. Aldawi, A.E. Anqi et al., Cost-effective and performance analysis of thermoelectricity as a building cooling system: experimental case study based on a single TEC-12706 commercial module. Case Stud. Therm. Eng. 27, 101366 (2021). https://doi.org/10.1016/j.csite.2021.101366
M.K.R. Alam, H. Fitriawan, F.X.A. Setyawan et al., Design of a cooling and heating tool using thermoelectric peltier based on Arduino Uno. Jurnal Teknik Elektro 13(1), 41–47 (2021)
D. Ponikvar, Experiments on temperature regulation using a Peltier element and PID technique. Eur. J. Phys. 43, 035809 (2022). https://doi.org/10.1088/1361-6404/ac5b1f/pdf
A. Kherkhar, Y. Chiba, A. Tlemçani et al., Thermal investigation of a thermoelectric cooler based on Arduino and PID control approach. CSITE 36, 102249 (2022)
P.L.K. Rao, The controller for the temperature chamber with Peltier cell. SVOČ 2023, p. 9 (2023). http://svoc.tul.cz/Reg/mechatronika/Pavan_FM_NMSP.pdf. Accessed 10 July 2023
Y. Liu, S. Yang, B. Guo et al., Numerical analysis and design of thermal management system for lithium ion battery pack using thermoelectric coolers. Adv. Mech. Eng. 2014, 852712 (2014). https://doi.org/10.1155/2014/852712
Hebei IT. Thermoelectric Cooler TEC1-12706 (2006). http://www.hebeiltd.com.cn/peltier.datasheet/TEC1-12706.pdf. Accessed 6 July 2023
Infineon Technologies. IR2110 500V high-side and low-gate driver IC with shutdown, p. 17 (2019). https://www.infineon.com/cms/en/product/power/gate-driver-ics/ir2110/. Accessed 10 July 2023
Infineon Technologies. IRLZ24N 55V Single N-Channel Power MOSFET in a TO-220 package. PD 94998, p. 10 (2021). https://www.infineon.com/cms/en/product/power/mosfet/n-channel/irlz24n/. Accessed 10 July 2023
R. Willem, H-Bridge Microchip PIC Microcontroller PWM Motor Controller (2009–2023). http://www.ermicro.com/blog/?p=706. Accessed 10 July 2023
A. Ostadfar, Biofluid Mechanics: Principles and Applications (Elsevier, Amsterdam, 2016). https://doi.org/10.1016/C2014-0-01583-3
Y. Mendelson, Biomedical Sensors. Chapter 10. in Introduction to Biomedical Engineering, 3rd edn. (Elsevier, Amsterdam, 2012), pp. 609–666. https://doi.org/10.1016/B978-0-12-374979-6.00010-1
A.Y.K. Chan, Biomedical Device Technology: Principles and Design, 3rd edn. (Springfiled, Charles C Thomas Pub Ltd, 2023), p.912
Dongguan Aolittel Electronics. Radial Leaded NTC 10k Thermistor Temperature Sensor MF51 (2021). https://ntc-sensors.com/product/en/Product-20211006-094839.html. Accessed 10 July 2023
Amphenol Sensors. Thermometrics Temperature Sensors in Catheter Applications, p. 2 (2021). https://www.amphenol-sensors.com/hubfs/AAS-930-292A-Thermometrics-Temp-Catheters-120821-web.pdf. Accessed 4 July 2023
Amphenol Corporation. Glass-Encapsulated Chip (GC) Thermistor, p. 1 (2018). https://f.hubspotusercontent40.net/hubfs/9035299/Documents/AAS-930-211A-Thermometrics-GC-Thermistor-062618-web.pdf. Accessed 4 July 2023
Amphenol Sensors. “AB” Thermistor for Healthcare, p. 1 (2015). https://media.digikey.com/pdf/Data%20Sheets/Thermometrics%20Global%20Business%20PDFs/AB-Thermistors.pdf. Accessed 4 July 2023
Amphenol Sensors. Type GC11 GC14, GC16 Thermometrics Glass Encapsulated NTC Chip Thermistor, p. 4 (2014). https://f.hubspotusercontent40.net/hubfs/9035299/Documents/AAS-920-584A-Thermometrics-Type-GC11-14-16-032114-web.pdf. Accessed 4 July 2023
TE Sensor Solution. Glass Bead Fast Time Response Probe, p. 4 (2015). https://www.te.com/usa-en/product-GAG22K7MCD419.html. Accessed 4 July 2023
Semitec Global. Medical Catalog, p. 1 (2022). https://www.semitec-global.com/uploads/2022/01/Medical.pdf. Accessed 4 July 2023
Semitec Global. Fμ Thermistor, p. 1 (2017). https://www.semitec-global.com/uploads/2022/01/P8-F-micro-Thermistor.pdf. Accessed 4 July 2023
Sensors Scientific Inc. Small Bead Thermistors, p. 2 (2018). https://irp-cdn.multiscreensite.com/e8db50c6/files/uploaded/GlassBead_NTC-0918.pdf. Accessed 10 July 2023
SourceCom Technology. Thermodilution Swan-Ganz Catheter, p. 7 (2015). http://www.sourcecomtech.com/wp-content/uploads/2015/07/Medical-Catheters-New.pdf. Accessed 10 July 2023
W. Bolton, Instrumentation and Control Systems, 3rd ed. Newnes, p. 392. (2021). https://doi.org/10.1016/C2020-0-00286-0
R. Teja, Wheatstone Bridge: Working, Examples, Applications (2021). https://www.electronicshub.org/wheatstone-bridge/. Accessed 10 July 2023
Texas Instruments. LMx58-N Low-Power, Dual-Operational Amplifiers, p. 41 (2023). https://www.ti.com/lit/ds/symlink/lm158-n.pdf. Accessed 5 July 2023
Texas Instruments. LM340, LM340A and LM7805 Family Wide VIN 1.5-A Fixed Voltage Regulators datasheet (Rev. L), p. 38 (2016). https://www.ti.com/product/LM7800. Accessed 7 July 2023
Microchip Technology. PIC16F631/677/685/687/689/690 DataSheet, p. 258 (2006). https://ww1.microchip.com/downloads/en/DeviceDoc/41262a.pdf. Accessed 10 July 2023
Ward, H.: C Programming for the PIC Microcontroller: Demystify Coding with Embedded Programming. Apress, p. 271. ISBN: 978-1-4842-5525-4. (2020). https://doi.org/10.1007/978-1-4842-5525-4
M. Predko, Programming and Customizing the PIC Microcontroller. McGraw-Hill, 3rd edn., p. 1293. ISBN-13: 978-0071472876 (2008). https://www.quillby.nl/vindigo/phocadownload/userupload/Prog.&Cust.PIC.pdf. Accessed 11 July 2023
H. Parchizadeh, B. Vukanovic, PIC Projects: A Practical Approach (Wiley, New York, 2009), p. 224. ISBN: 978-0-470-69461-9
D.W. Smith, PIC Projects and Applications using C. Elsevier Newnes 2013, p. 183. (2013). https://doi.org/10.1016/C2013-0-00524-5
C.L. Izidoro, Desenvolvimento de uma bancada didática para estudos dos efeitos termoelétricos aplicados na engenharia, p. 140 (2015). https://lume.ufrgs.br/handle/10183/130128. Accessed 15 July 2023
Microchip Developer Help. Analog-to-Digital Converter (2021). https://microchipdeveloper.com/8bit:adc. Accessed 10 July 2023
T. Steffes, M. Smith, M. Brehob, EABLE PCB Introduction. Univ of Michigan, p. 19 (2019). https://www.eecs.umich.edu/courses/eecs473/Labs/EAGLE_PCB_Introduction.pdf. Accessed 10 July 2023
C. Yang, EAGLE CAD Tutorial. ECE 445, Univ of Illinois, p. 20 (2016). https://courses.engr.illinois.edu/ece445/documents/EaglecadTutorial.pdf. Accessed 10 July 2023
F. Li, PCB Design with Eagle. ECE, Univ of Idaho, p. 64 (2014). https://www.webpages.uidaho.edu/mindworks/Capstone%20Design/Presentations/FLi%20-%20PCB%20Design%20with%20EAGLE.PDF. Accessed 10 July 2023
CadSoft Computer GmbH. EAGLE Easily Applicable Graphical Layout Editor. Tutorial v.6, 1 ed, p. 68 (2011). https://www.egr.msu.edu/eceshop/pcb/V6_tutorial_en.pdf. Accessed 10 July 2023
Texas Instruments. MAX232x Dual EIA-232 Drivers/Receivers, p. 28 (2014–2023). https://www.ti.com/lit/ds/symlink/max232.pdf. Accessed 10 July 2023
M. Kapoor, M. Stone, Cardiac output and intravascular volume in Monitoring in Anesthesia and Perioperative Care (Cambridge University, Cambridge, 2011), pp. 79–94. https://doi.org/10.1017/CBO9780511974083.010
B.F. Geerts, L.P. Aarts, J.R. Jansen, Methods in pharmacology: measurement of cardiac output. Br. J. Clin. Pharmacol. 71(3), 316–330 (2010). https://doi.org/10.1111/j.1365-2125.2010.03798.x
S.G. Sakka, D.A. Reuter, A. Perel, The transpulmonary thermodilution technique. J. Clin. Monit. Comput. 26, 347–353 (2012). https://doi.org/10.1007/s10877-012-9378-5
C. Chamos, L. Vele, M. Hamilton et al., Less invasive methods of advanced hemodynamic monitoring: principles, devices, and their role in the perioperative hemodynamic optimization. Med. Perioper. (2013). https://doi.org/10.1186/2047-0525-2-19
Unisinos. http://www.unisinos.br/global/en/. Accessed 10 July 2023
V. Hillmann, An Introduction to C++ Builder 2010, p. 106 (2009). https://www.embarcadero.com/images/dm/technical-papers/introduction-to-cppbuilder-whitepaper.pdf. Accessed 11 June 2023
S. Meyers, Effective Modern C++: 42 Specific Ways to Improve Your Use of C++ 11 and C++ 14, 1st edn. (O’Reilly Media, 2014), p. 451
B. Stroustrup, The C++ Programming Language, 4th edn (Addison-Wesley, Upper Saddle River, 2021), p. 1368. https://www.stroustrup.com/4th.html. Accessed 18 July 2023
Edwards Lifesciences Corporation. Swan-Ganz Catheter Computation Constants. PP-US-2259 v3.0, p. 2 (2023). https://education.edwards.com/computation-constants/94554#swan-ganz. Accessed 11 July 2023
J. Balakrishnan (2017) Approximating area under curves and Riemann sums. Boston University, Depart. Math & Statistics. http://math.bu.edu/people/jbala/teaching/20171116.pdf. Accessed 11 July 2023
Santa Casa de Misericórdia of Porto Alegre. International website. https://www.santacasa.org.br/pagina/international Accessed Jul 2023
R. Boylestad, L. Nashelsky, Electronic Devices and Circuit Theory (Pearson Education, 2021), p. 927. ISBN 978-0-13-262226-4
YSI Incorporated. Instructions for YSI Series 400 Temperature Probes (2012). https://neurophysics.ucsd.edu/Manuals/YSI/YSI%20Series%20400%20Temperature%20Probe.pdf. Accessed 20 Sept 2023
Dixtal. Manual de Operação—Monitor DX 2010. Capítulo sobre débito cardíaco. Revisão (2007)
W. Rutala, et al., Guideline for Disinfection and Sterilization in Healthcare Facilities. Centers for Disease Control and Prevention, p. 163 (2008). https://www.cdc.gov/infectioncontrol/guidelines/disinfection/. Accessed 11 July 2023
B. Johnson, M. Adi, M.G. Licina, et al., Chapter 3—Cardiac Physiology. in: Essentials of Cardiac Anesthesia, ed. J. Kaplan (Elsevier, Amsterdam, 2009), pp.53–66. https://doi.org/10.1016/B978-141603786-6.10003-8
J. King, D.R. Lowery, Physiology, Cardiac Output (2022). https://www.ncbi.nlm.nih.gov/books/NBK470455/. Accessed 11 July 2023
Clean Medical. DX2023 Monitor de Sinais Vitais—Manual de Operação, p. 54 (2019). http://cleanmedical.com.br/wp-content/uploads/2019/12/DIXTAL-2023.pdf. Accessed 11 July 2023
Strategic Market Research. Hemodynamic Monitoring Systems Market Size, Report 2030 (2022). https://www.strategicmarketresearch.com/market-report/hemodynamic-monitoring-market. Accessed 11 July 2023
J.J. Darrow, J. Avorn, A.S. Kesselheim, FDA regulation and approval of medical devices: 1976–2020. JAMA 326(5), 420–432 (2021). https://doi.org/10.1001/jama.2021.11171
E. Mallis, An Introduction to FDA’s Regulation of Medical Devices. US Food and Drug Administration (2019). https://www.fda.gov/media/123602/download. Accessed 17 July 2023
M.M. Brigmon, R.L. Brigmon, Infectious diseases impact on biomedical devices and materials. Biomed. Mater. Dev. (2022). https://doi.org/10.1007/s44174-022-00035-y
R. Narayan (ed.), in Biomedical Materials (Springer, Cham, 2021), p. 728. https://doi.org/10.1007/978-3-030-49206-9
Applied Thermoelectric Solutions. Listing of Thermoelectric Companies, Manufacturers, Suppliers—TEG and Cooling (2023). https://thermoelectricsolutions.com/list-of-thermoelectric-peltier-manufactures-companies-suppliers/. Accessed 17 July 2023
Advanced Thermal Solutions Inc. How to Select a Thermoelectric Cooler, p. 3 (2014). https://www.qats.com/Download/Qpedia_May07_TEC_Selection5.ashx. Accessed 17 July 2023
Crystal Ltd. Thermoelectric Modules Catalogue, p. 16 (2020). https://crystalltherm.com/images/Katalogi/Crystal_catalogue_TEM.pdf. Accessed 17 July 2023
CUI Devices Inc. Peltier Application Note, p. 9 (2019). https://www.cuidevices.com/catalog/resource/peltier-app-note.pdf. Accessed 11 June 2023
CUI Devices Inc. Peltier Devices (2023). https://www.cuidevices.com/catalog/thermal-management/peltier-devices. Accessed 17 July 2023
European Thermodynamics Ltd. Thermoelectric Modules—Thermoelectric Cooler Modules (2020). https://www.europeanthermodynamics.com/products/thermoelectric-modules/peltier-cooler. Accessed 17 June 2023
Ferrotec Corporation. 9.0 Thermoelectric Module Selection (2023). https://thermal.ferrotec.com/technology/thermoelectric-reference-guide/thermalref09/. Accessed 17 Jul, 2023
Hebeit IT Shanghai. Peltier Thermoelectric Cooling Modules, p. 3 (2014). https://peltiermodules.com/peltier.datasheet/Peltier_Modules.pdf. Accessed 17 June 2023
Laird Thermal Systems Inc. Thermoelectric Coolers Catalog, p. 8 (2022). https://lairdthermal.com/sites/default/files/ckfinder/files/resources/Catalogs/Thermoelectric-Modules/Thermoelectric-Coolers-Catalog-011822.pdf. Accessed 17 June 2023
TEC Microsystems GmbH. Thermoelectric Coolers Frequently Asked Questions, p. 91 (2018). https://www.tec-microsystems.com/Download/Docs/TEC_FAQ_TCM.pdf. Accessed 17 June 2023
TEC Microsystems GmbH. 1MA10 Series Aluminum TEC Intro, p. 9 (2020). https://www.tec-microsystems.com/Download/Docs/1MA10_Series_Aluminium_TECs_Intro.pdf. Accessed 11 Aug 2023
Meerstetter Engineering. TEC/Peltier Element Design Guide (2023). https://www.meerstetter.ch/customer-center/compendium/32-tec-peltier-element-design-guide. Accessed 11 July 2023
A. Pressman, Switching Power Supply Design, 2nd edn. (McGraw Hill, New York, 1997), p.682
P. Horowitz, W. Hill, The Art of Electronics, 3rd edn. (Cambridge University Press, Cambridge, 2015), p.1220
P. Scherz, S. Monk, Practical Electronics for Inventors, 4th edn. (McGraw Hill, New York, 2016), p.1056
R.W. Erickson, D. Maksimović, Fundamentals of Power Electronics, 3rd edn. (Springer, New York, 2020), p.1103
Ferrotec Corporation. Thermoelectric Technical Reference—7.0 Power Supply Requirements (2023). https://thermal.ferrotec.com/technology/thermoelectric-reference-guide/thermalref07/. Accessed 6 Sept 2023
X. Guo, M.A. Khalid, I. Domingos et al., Smartphone-based DNA diagnostics for malaria detection using deep learning for local decision support and blockchain technology for security. Nat. Electron. 4, 615–624 (2021). https://doi.org/10.1038/s41928-021-00612-x
J.W.T.M. de Kok et al., A guide to sharing open healthcare data under the General Data Protection Regulation. Sci. Data 10, 404 (2023). https://doi.org/10.1038/s41597-023-02256-2
World Health Organization. The protection of personal data in health information systems—principles and processes for public health, p. 35 (2021). https://apps.who.int/iris/bitstream/handle/10665/341374/WHO-EURO-2021-1994-41749-57154-eng.pdf?sequence=1&isAllowed=y. Accessed 11 July 2023
Information Commissioner’s Office. Guide to the General Data Protection Regulation (GDPR), p. 295 (2018). https://ico.org.uk/media/for-organisations/guide-to-the-general-data-protection-regulation-gdpr-1-0.pdf. Accessed 18 July 2023
W. Zayat, O. Senvar, Framework study for Agile software development via Scrum and Kanban. Int. J. Innov. Technol. Manag. 17(4), 2030002 (2020). https://doi.org/10.1142/S0219877020300025
C. Hofmann, S. Lauber, B. Haefner, G. Lanza, Development of an agile software development method based on Kanban for distributed part-time teams and an introduction framework. Procedia Manuf. 23, 45–50 (2018). https://doi.org/10.1016/j.promfg.2018.03.159
S. Al-Saqqa, S. Sawalha, H. AbdelNabi, Agile software development: methodologies and trends. IJIM 14(11), 246–270 (2020). https://doi.org/10.3991/ijim.v14i11.13269
Alpha Medical Instruments LLC. Thermodilution Catheter—Series 400 (2015). http://www.alphamedicalinstruments.com/pdf/Thermodilution_Catheter.pdf. Accessed 11 July 2023
Alpha Medical Instruments LLC. Thermodilution Infusion Catheter—Series 500, p. 2 (2015). http://www.alphamedicalinstruments.com/pdf/Thermodilution_Infusion_Catheter.pdf. Accessed 11 July 2023
Bioptimal International PTE. Instructions for use of thermodilution catheters and kits, p. 2 (2020). https://www.bioptimalg.com/Files/TD%E8%8B%B1%E6%96%87-51-000014-00_J2_200430.pdf. Accessed 10 July 2023
Bioptimal International PTE. Thermodilution Catheter—Pulmonary Artery Monitoring Catheter and Biotray, p. 2 (2021). https://www.bioptimalg.com/Files/2.%20TD-%20PA.pdf. Accessed 10 July 2023
Biosensors International. SafetyWedge Thermodilution Catheter (2012). https://biosensors.com/intl/sites/default/files/pdfs/products_technology/safety_wedge.pdf. Accessed 11 July 2023
B. Braun SE. Right Heart Catheter (2023). https://www.bbraun.com/en/products/b/right-heart-catheter.html. Accessed 11 July 2023
B. Braun SE. Hemodynamics Brochure, p. 48 (2017). https://www.bbraun-vetcare.es/content/dam/b-braun/es/microsite/informacion-de-producto/anestesia-y-analgesia/6050166%20Hemodynamics.pdf. Accessed 11 July 2023
DeRoyal Industries. Thermodilution catheters (2023). https://www.deroyal.com/products/search-catalog-item/catalog-item-preview/ac-cathlab-thermodilutecath. Accessed 10 July 2023
Edwards Lifesciences Corporation. Advanced Hemodynamic Monitoring Swan-Ganz Pulmonary Artery Catheters. Brochure PP-US-3089 v2. https://education.edwards.com/swan-ganztm-poster/258441#swan-ganz# (2018). Accessed 11 July 2023.
Edwards Lifesciences Corporation. Critical Care Product Catalog. PP-US-1728 v5.0, p. 55 (2023). https://education.edwards.com/critical-care-product-catalog/653191#. Accessed 10 July 2023
ICU Medical Incorporated. Pulmonary Artery Catheters—With No Natural Rubber Latex Components. M1-1244 Rev. 05, p. 4 (2021). https://www.icumed.com/media/14797/m1-1244-pa-cath-latex-free-rev-05_ada_web.pdf. Accessed 11 July 2023
intra special catheters GmbH. Products, p. 30 (2021). https://www.intra-online.de/neu/pdf/2/2021_intra.pdf. Accessed 11 July 2023
KFF S.A. Thermodilution Catheter. 2 p. https://kffmed.com/wp-content/uploads/2018/04/Binder1.pdf (2021). Accessed 15 Aug 2023.
Merit Medical Systems. Flow-directed thermodilution catheter: instruction for use, p. 88 (2022). https://www.merit.com/wp-content/uploads/2022/09/51-000014-05_REV-A_.indd-TD.pdf. Accessed 11 July 2023
Merit Medical Systems. Merit Pulmonary Artery and Thermodilution Catheters, p. 2 (2022). https://cloud.merit.com/catalog/Brochures/406342001.pdf. Accessed 11 July 2023
Nipro Canada. Thermodilution catheter, p. 2 (2019). https://nipro.ca/wp-content/uploads/2019/10/CC_ThermoDilutionCath_Rev4.pdf. Accessed 10 July 2023
Teleflex Inc. Arrow® Right Heart Catheters and Vascular Access Sheaths: Diagnostic Monitoring and Therapeutic Catheter Solutions, p. 9 (2021). https://www.teleflex.com/usa/en/product-areas/interventional/cardiac-diagnostics/arrow-thermodilution-catheters/CC_RH_Right-Heart-Product-Brochure_BR_MC-000166_Rev%203_final.pdf. Accessed 17 July 2023
Zeon Corporation. Thermodilution catheter, p. 2 (2019). https://www.zeonmedical.co.jp/product/circulation/pdf/TDC_TPC_W_1905_ver02_out.pdf. Accessed 16 Aug 2023
J.M. Bland, D.G. Altman, Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet 327(8476), 307–310 (1986). https://doi.org/10.1016/S0140-6736(86)90837-8
D.G. Altman, J.M. Bland, Measurement in medicine: the analysis of method comparison studies. Statistician 32(3), 307–317 (1983). https://doi.org/10.2307/2987937
D. Giavarina, Understanding Bland Altman analysis. Biochemia Medica 25(2), 141–151 (2015). https://doi.org/10.11613/BM.2015.015
C.A. Willard, An Introduction to Basic Statistical Concepts and Analysis, 2nd edn. (Routledge, London, 2020), p.364. https://doi.org/10.4324/9780429261039
K.F. Bachmann, L. Zwicker, K. Nettelbeck, D. Casoni, P.P. Heinisch, H. Jenni, M. Haenggi, D. Berger, Assessment of right heart function during extracorporeal therapy by modified thermodilution in a porcine model. Anesthesiology 133(4), 879–891 (2020). https://doi.org/10.1097/ALN.0000000000003443
N. Kiefer, C.K. Hofer, G. Marx, M. Geisen, R. Giraud, N. Siegenthaler, A. Hoeft, K. Bendjelid, S. Rex, Clinical validation of a new thermodilution system for the assessment of cardiac output and volumetric parameters. Crit. Care 16(3), R98 (2012). https://doi.org/10.1186/cc11366
S.T. Heerwagen, L. Lönn, T.V. Schroeder et al., Catheter-based flow measurements in hemodialysis fistulas—bench testing and clinical performance. J. Vasc. Access 13(1), 45–50 (2012). https://doi.org/10.5301/JVA.2011.8443
D. Furlong, D.L. Carroll, C. Finn et al., Comparison of temporal to pulmonary artery temperature in febrile patients. Dimens. Crit. Care Nurs. 34(1), 47–52 (2015). https://doi.org/10.1097/DCC.0000000000000090
F. Cipulli, M. Battistin, E. Carlesso et al., Quantification of recirculation during veno-venous extracorporeal membrane oxygenation: in vitro evaluation of a thermodilution technique. ASAIO J. 68(2), 184–189 (2022)
I. Gratz, M. Baruch, A. Awad et al., A new continuous noninvasive finger cuff device (Vitalstream) for cardiac output that communicates wirelessly via bluetooth or Wi-Fi. BMC Anesthesiol. 23(1), 180 (2023). https://doi.org/10.1186/s12871-023-02114-z
A. Dvir, N. Goldstein, A. Rapaport et al., Comparing cardiac output measurements using a wearable, wireless, noninvasive photoplethysmography-based device to pulse contour cardiac output in the general ICU: a brief report. Crit. Care Explor. 4(2), e0624 (2022)
S.D. Gregory, H. Cooney, S. Diab et al., In vitro evaluation of an ultrasonic cardiac output monitoring (USCOM) device. J. Clin. Monit. Comput. 30(1), 69–75 (2016). https://doi.org/10.1007/s10877-015-9685-8
Y. Launey, R. Larmet, N. Nesseler, Y. Malledant, C. Palpacuer, P. Seguin, The accuracy of temperature measurements provided by the Edwards lifesciences pulmonary artery catheter. Anesth. Analg. 122(5), 1480–1483 (2016). https://doi.org/10.1213/ANE.0000000000001242
L. Mitrev, N. van Helmond, G. Kaddissi et al., A pilot study comparing aortic valve area estimates derived from Fick cardiac output with estimates based on Cheetah-NICOM cardiac output. Sci. Rep. 10, 7852 (2020). https://doi.org/10.1038/s41598-020-64753-3
M. Russ, E. Steiner, W. Boemke et al., Extracorporeal membrane oxygenation blood flow and blood recirculation compromise thermodilution-based measurements of cardiac output. ASAIO J. 68(5), 721–729 (2022). https://doi.org/10.1097/MAT.0000000000001592
A.J. Milam, F. Ghoddoussi, J. Lucaj, S. Narreddy, N. Kumar, V. Reddy, J. Hakim, S.H. Krishnan, Comparing the mutual interchangeability of ECOM, FloTrac/Vigileo, 3D-TEE, and ITD-PAC cardiac output measuring systems in coronary artery bypass grafting. J. Cardiothorac. Vasc. Anesth. 35(2), 514–529 (2021). https://doi.org/10.1053/j.jvca.2020.03.048
F. Tettey, S.K. Parupelli, S. Desai, A review of biomedical devices: classification, regulatory guidelines, human factors, software as a medical device, and cybersecurity. Biomed. Mater. Dev. (2023). https://doi.org/10.1007/s44174-023-00113-9
C.L. Sprung, L.A. Eidelman, The issue of a U.S. Food and Drug Administration moratorium on the use of the pulmonary artery catheter. New Horiz. 5(3), 277–280 (1997)
G.R. Bernard, G. Sopko, F. Cerra et al., Pulmonary artery catheterization and clinical outcomes. JAMA 283(19), 2568–2572 (2000). https://doi.org/10.1001/jama.283.19.2568
R.M. Perkin, N. Anas, Pulmonary artery catheters. Pediatr. Crit. Care Med. 12(4 Suppl), S12–S20 (2011). https://doi.org/10.1097/PCC.0b013e318220f079
K. Piermatteo, In: U.S. Food and Drug Administration. The 510(k) Program, p. 67 (2014). https://www.fda.gov/media/89869/download. Accessed 10 Aug 2023
I. Abuhav, ISO 13485: 2016—A Complete Guide to Quality Management in the Medical Device Industry (CRC Press, Boca Raton, 2018), p.893
International Organization for Standardization. ISO 13485—Quality management for medical devices, p. 12. https://www.iso.org/files/live/sites/isoorg/files/store/en/PUB100377.pdf(2016). Accessed 11 July 2023
U.S. Food and Drug Administration. 510(k) Clearances (2021). https://www.fda.gov/medical-devices/device-approvals-denials-and-clearances/510k-clearances. Accessed 10 Aug 2023
U.S. Food and Drug Administration. Premarket Notification 510(k) (2022). https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-notification-510k. Accessed 10 Aug 2023
Center for Devices and Radiological Health. Electromagnetic Compability (EMC) of Medical Devices: Guidance for Industry and Food and Drug Administration Staff, p. 20 (2022). https://www.fda.gov/media/94758/download. Accessed 11 July 2023
I. Juuso, Developing an ISO 13485-Certified Quality Management System: An Implementation Guide for the Medical-Device Industry (Routledge/Productivity Press, Taylor & Francis Group, London, 2022), p.371. https://doi.org/10.4324/9781003202868
C.R. Paul, R.C. Scully, M.A. Steffka, Introduction to Electromagnetic Compatibility, 3rd edn. (Wiley, New York, 2022), p.848
J. Wong, R.K.Y. Tong, Medical Regulatory Affairs: An International Handbook for Medical Devices and Healthcare Products (3rd ed.). (Jenny Stanford Publishing, 2022), p. 806. https://doi.org/10.1201/9781003207696
A.K. Sarata, FDA Regulation of Medical Devices. Congressional Research Service R47374, p. 45. Jan 4, 2023 (2023). https://crsreports.congress.gov/product/pdf/R/R47374. Accessed 12 Aug 2023
T.J. Iberti, E.K. Daily, A.B. Leibowitz et al., Assessment of critical care nurses’ knowledge of the pulmonary artery catheter. The Pulmonary Artery Catheter Study Group. Crit. Care Med. 22(10), 1674–1678 (1994)
A. Gnaegi, F. Feihl, C. Perret, Intensive care physicians’ insufficient knowledge of right-heart catheterization at the bedside: time to act? Crit. Care Med. 25(2), 213–220 (1997)
M.L. Good, Patient simulation for training basic and advanced clinical skills. Med. Educ. 37(Suppl 1), 14–21 (2003). https://doi.org/10.1046/j.1365-2923.37.s1.6.x
A.B. Al-Elq, Simulation-based medical teaching and learning. J. Fam. Community Med. 17(1), 35–40 (2010). https://doi.org/10.4103/1319-1683.68787
D.M. Harris, K. Ryan, C. Rabuck, Using a high-fidelity patient simulator with first-year medical students to facilitate learning of cardiovascular function curves. Adv. Physiol. Educ. 36(3), 213–219 (2012). https://doi.org/10.1152/advan.00058.2012
I. Motola, L.A. Devine, H.S. Chung et al., Simulation in healthcare education: a best evidence practical guide. AMEE Guide No. 82. Med. Teach. 35(10), e1511-1530 (2013). https://doi.org/10.3109/0142159X.2013.818632
H.Y. So, P.P. Chen, G.K.C. Wong et al., Simulation in medical education. J. R. Coll. Physicians Edinb. 49(1), 52–57 (2019). https://doi.org/10.4997/jrcpe.2019.112
L.J. Davidson, K.Y. Chow, A. Jivan et al., Improving cardiology fellow education of right heart catheterization using a simulation based curriculum. Catheter. Cardiov. Interv. 97(3), 503–508 (2021). https://doi.org/10.1002/ccd.29128
M.H. Tukey, R.S. Wiener, The current state of fellowship training in pulmonary artery catheter placement and data interpretation: a national survey of pulmonary and critical care fellowship program directors. J. Crit. Care 28(5), 857–861 (2013). https://doi.org/10.1016/j.jcrc.2013.06.003
D. Chaló, J. Marques, H. Mendes et al., Design of an interface for teaching cardiovascular physiology to anesthesia clinicians with a patient simulator connected to a minimally invasive cardiac output monitor (LiDCO rapid®). Adv. Simul. 5, 16 (2020). https://doi.org/10.1186/s41077-020-00134-0
Clinical Gate. Hemodynamic Monitoring (2015). https://clinicalgate.com/hemodynamic-monitoring-3/. Accessed 10 July 2023
Respiratory Update. Pulmonary Artery Catheter: Types, Uses, Contraindications (2023). http://www.respiratoryupdate.com/members/PA_Catheter_Types.cfm. Accessed 11 July 2023
S. Sigh, S. Sharma, High-Output Cardiac Failure (2022). https://www.ncbi.nlm.nih.gov/books/NBK513337/. Accessed 11 July 2023.
Acknowledgements
The authors thank the ISCMPA for providing the medical equipment for prototype validation tests. The authors thank Unisinos University and Fundação Liberato for providing access to the laboratories necessary for developing and adjusting the hardware and software of this research. The authors thank J. Larsen, T. Adamson, M. Delgado, D. Bertolozi, and J. Neto for providing comments that brought improvements to the manuscript. Last but not least, the authors are grateful for the valuable help and guidance of Professor A. Lawisch since day one and the support of our families who were with us on this journey.
Author information
Authors and Affiliations
Contributions
CFTdA participated in the conceptualization; investigation; hardware and software design, writing of the original draft and reviewing and editing of the manuscript; data curation; methodology; data analysis and discussion; and final considerations. BTdA participated in the writing, reviewing, and editing of the manuscript; methodology reviewing; and data analysis reviewing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict and non-competing interests for developing this research.
Ethical Approval
The final tests of the prototype conducted in this research were approved by the Ethical Committee and Coordination of Biomedical Engineering Department of the ISCMPA. The authors followed all the guidelines, legislation, legal and ethical standards for use of the medical equipment.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Abreu, C.F.T., de Abreu, B.T. The Concept and Building of a Simulation Device to Check the Cardiac Output Measurement Through the Pulmonary Artery Catheter. Biomedical Materials & Devices (2023). https://doi.org/10.1007/s44174-023-00130-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44174-023-00130-8