Abstract
Aims/hypothesis
Oxylipins are lipid mediators derived from polyunsaturated fatty acids. Some oxylipins are proinflammatory (e.g. those derived from arachidonic acid [ARA]), others are pro-resolving of inflammation (e.g. those derived from α-linolenic acid [ALA], docosahexaenoic acid [DHA] and eicosapentaenoic acid [EPA]) and others may be both (e.g. those derived from linoleic acid [LA]). The goal of this study was to examine whether oxylipins are associated with incident type 1 diabetes.
Methods
We conducted a nested case–control analysis in the Diabetes Autoimmunity Study in the Young (DAISY), a prospective cohort study of children at risk of type 1 diabetes. Plasma levels of 14 ARA-derived oxylipins, ten LA-derived oxylipins, six ALA-derived oxylipins, four DHA-derived oxylipins and two EPA-related oxylipins were measured by ultra-HPLC–MS/MS at multiple timepoints related to autoantibody seroconversion in 72 type 1 diabetes cases and 71 control participants, which were frequency matched on age at autoantibody seroconversion (of the case), ethnicity and sample availability. Linear mixed models were used to obtain an age-adjusted mean of each oxylipin prior to type 1 diabetes. Age-adjusted mean oxylipins were tested for association with type 1 diabetes using logistic regression, adjusting for the high risk HLA genotype HLA-DR3/4,DQB1*0302. We also performed principal component analysis of the oxylipins and tested principal components (PCs) for association with type 1 diabetes. Finally, to investigate potential critical timepoints, we examined the association of oxylipins measured before and after autoantibody seroconversion (of the cases) using PCs of the oxylipins at those visits.
Results
The ARA-related oxylipin 5-HETE was associated with increased type 1 diabetes risk. Five LA-related oxylipins, two ALA-related oxylipins and one DHA-related oxylipin were associated with decreased type 1 diabetes risk. A profile of elevated LA- and ALA-related oxylipins (PC1) was associated with decreased type 1 diabetes risk (OR 0.61; 95% CI 0.40, 0.94). A profile of elevated ARA-related oxylipins (PC2) was associated with increased diabetes risk (OR 1.53; 95% CI 1.03, 2.29). A critical timepoint analysis showed type 1 diabetes was associated with a high ARA-related oxylipin profile at post-autoantibody-seroconversion but not pre-seroconversion.
Conclusions/interpretation
The protective association of higher LA- and ALA-related oxylipins demonstrates the importance of both inflammation promotion and resolution in type 1 diabetes. Proinflammatory ARA-related oxylipins may play an important role once the autoimmune process has begun.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Type 1 diabetes is an autoimmune disease characterised by development of islet autoimmunity (IA) and associated pancreatic islet cell inflammation [1]. In addition to genetic susceptibility for type 1 diabetes, most strongly represented by the HLA region [2], environmental factors have been hypothesised to play a role in the development of type 1 diabetes [3]. Polyunsaturated fatty acids (FAs), specifically n-3 and n-6 FAs, may play a role in the development of type 1 diabetes, potentially related to their integral role in inflammation. While n-3 FAs are generally considered anti-inflammatory [4], n-6 FAs are generally pro-inflammatory [5]. In the Diabetes Autoimmunity Study in the Young (DAISY), we previously found that higher n-3 levels in erythrocyte membrane were associated with a decreased risk of IA [6, 7]. Because of their opposing actions, an imbalance in the n-6:n-3 intake may lead to increased inflammation [8]. Indeed, the Finnish Type 1 Diabetes Prediction and Prevention Study (DIPP) birth cohort showed that elevated plasma arachidonic acid (ARA, an n-6 FA) to docosahexaenoic acid (DHA, an n-3 FA) ratios were associated with increased risk of IA [9].
The role of n-6 and n-3 FAs in inflammation is related to the production of oxylipins, which are oxygenated lipid mediators that promote or resolve inflammation. The n-6-related oxylipins are generally considered to be more inflammatory, proliferative and vasoconstrictive, compared with n-3 oxylipins, although oxylipins derived from the n-6 FA linoleic acid (LA) demonstrate some anti-inflammatory effects [10]. The n-3-related oxylipins generally are anti-inflammatory, anti-proliferative and pro-resolving [10]. Imbalances in n-6 and n-3 oxylipins have been found in patients with the autoimmune conditions ulcerative colitis [11] and arthritis [8, 12, 13]. In healthy DAISY participants without type 1 diabetes, we found that most oxylipins were significantly associated with their precursor FA (e.g. LA, ARA, DHA) measured in plasma [14]. Proportionally more n-3- compared with n-6-related oxylipins were associated with their precursor FA measured in erythrocytes [14]. We also found that most oxylipins have a linear relationship with age.
No studies have examined oxylipins in relation to risk of type 1 diabetes. However, multiple studies have demonstrated the relationship between inflammatory markers and type 1 diabetes. For example, the inflammatory cytokine C-X-C motif chemokine ligand 10 may participate in insulitis and the destruction of islet cells [15]. IL-1β is another inflammatory cytokine that plays a role in damage to pancreatic islet cells [16]. The cytokine profile is different between those recently diagnosed with type 1 diabetes and healthy control participants [17]. Most studies focus on proinflammatory markers such as IFN-γ, TNF-α and IL-1β [18]. However, pro-resolving inflammatory markers may also be involved in the reduction in risk of type 1 diabetes. Oxylipins are unique biomarkers that represent both pro-inflammatory and pro-resolving pathways. The goal of this study was to test the association between plasma oxylipin levels and development of type 1 diabetes in high-risk children.
Methods
Study participants
DAISY is a longitudinal cohort study in Denver, CO, USA following 2547 children at risk of type 1 diabetes (Fig. 1). DAISY participants were recruited from the following two populations:
-
1.
Children from the general population born in 1993–2006 at the St Joseph’s Hospital in Denver, CO, USA without major neonatal morbidities who, through screening by umbilical cord blood for diabetes susceptibility alleles in the HLA region, were identified to have an increased risk for the disease. Of 31,766 screened newborns (87% of eligible children), 1424 were enrolled in the cohort.
-
2.
Children with a first-degree relative with type 1 diabetes in the Denver metropolitan area. A total of 1123 children were recruited irrespective of their HLA genotype.
DAISY study design. A nested case–control study was selected from the DAISY cohort of IA cases and IA control participants that were frequency matched to the case on age at autoantibody seroconversion (of the case), ethnicity and sample availability. A nested case–control study for the outcome of type 1 diabetes (T1D) was selected from the IA cases and control participants with available oxylipin measures. In the nested type 1 diabetes case–control study, we selected the visit prior to IA seroconversion as the pre-SV critical timepoint and the visit most immediately after seroconversion as the post-SV critical timepoint. Circles are not to scale
Participants’ study visits occurred at 9, 15 and 24 months, and then annually. Radio-immunoassays were used to measure serum autoantibodies to insulin, GAD autoantibodies (GADA), insulin autoantibodies (IAA) and insulinoma-associated-2 autoantibodies (IA-2), as described previously [19]. Any participants that tested positive for GADA, IAA or IA-2 were later tested for zinc transporter 8 autoantibodies (ZnT8A) as described previously [20]. IA was defined as testing positive for one or more islet autoantibodies on two or more consecutive visits or testing positive for islet autoantibodies once and being diagnosed with type 1 diabetes within a year using ADA criteria [21]. Parental consent was obtained for all participants, and assent was obtained from children ≥7 years of age. This study has been approved by the Colorado Multiple Institutional Review Board.
We used a nested case–control study of 343 children (171 IA-positive and 172 IA-negative children) within the DAISY cohort to first characterise oxylipin levels and profiles throughout childhood. The availability of this IA case–control population afforded us a larger group of at-risk individuals with which we could characterise and summarise the longitudinal oxylipin levels with more precision for each participant. Once these values were obtained, we selected 72 type 1 diabetes cases and 71 control participants from within this larger IA case–control population in order to test our hypothesis that oxylipins and their profiles are associated with the development of type 1 diabetes (Fig. 1). Control participants were frequency matched to the type 1 diabetes cases on age at autoantibody seroconversion (of the case) ±2 years, ethnicity and sample availability.
Measurement of oxylipins
Blood was drawn at study visits, and plasma was immediately separated then snap-frozen in liquid nitrogen and stored at −70°C. Blood draws were non-fasting. Oxylipin quantification has been described in detail by Pedersen and Newman [22]. Extracted oxylipins were quantified using a Waters i-Class Acquity ultra-HPLC system (Waters; Milford, MA, USA) coupled to a Sciex 6500+ QTRAP mass spectrometer (Sciex; Redwood City, CA, USA) operated in negative ionisation mode. Quantification of oxylipins was done by targeted, retention time-specific, multiple reaction monitoring (MRM) ion transitions.
As described previously, we measured n-6-related oxylipins, including ARA-related and LA-related oxylipins, and n-3-related oxylipins, which included α-linolenic acid (ALA)-related, DHA-related and eicosapentaenoic acid (EPA)-related oxylipins [14]. Oxylipins measured in a sample below the limit of quantification (LOQ), defined as signal-to-noise ratio below 3:1, were converted to 10% of the LOQ. Full names of the measured oxylipins are listed in electronic supplementary material (ESM) Table 1.
Oxylipins were measured in plasma samples from a maximum of four study visits in 343 children based around IA seroconversion: (1) The earliest sample available, which was between 9 and 15 months of age. (2) The sample collected just before IA seroconversion (pre-seroconversion [pre-SV]). (3) The sample collected just after IA seroconversion (post-seroconversion [post-SV]) (i.e. the first sample positive for autoantibodies). (4) The last sample available prior to type 1 diabetes diagnosis (in those IA-positive children that went on to develop type 1 diabetes) or a sample in an IA-negative child of a similar age. All oxylipin variables were Box–Cox transformed for normality. After Box–Cox transformation of 15-HEPE and lipoxin A4, the distribution of these variables was bimodal; therefore, we dichotomised these variables as present (levels above the LOQ) or absent (levels below the LOQ).
Mean oxylipin measure
In order to create a single variable that reflected the mean level of each oxylipin over these multiple timepoints, we used a linear mixed model with a random intercept and fixed slope using an unstructured covariance structure, with the Box–Cox-transformed oxylipins as the outcome and age as the predictor in the 343 children with oxylipin measurements. We used all individuals in whom oxylipins were measured for this analysis in order to improve the precision of the estimates for age-adjusted mean oxylipin levels. The participant-specific intercept of each oxylipin was used as an age-adjusted mean measure of the oxylipin. The model did not converge for three ARA-related oxylipins (PGE2, 6-trans-LTB4 and PGD2), so these were not included in subsequent analyses.
Principal component analysis of the mean oxylipins
Oxylipins share FA precursors and enzymes (see Fig. 2); therefore, they are unlikely to be independent of one another, i.e. a change in one is likely to be accompanied by a change in another. Because single oxylipin–outcome analytic approaches do not capture these interconnected networks of multiple oxylipins that may indicate shared and distinct inflammation resolution pathways, we conducted a principal component analysis (PCA) of the oxylipins in the 343 children with oxylipin measurements to find correlations between multiple oxylipins and group them into uncorrelated principal components (PCs) for analysis. Similarly, for the mean oxylipin measures, we used all children in whom oxylipins were measured in order to improve the precision of the measurement of the oxylipin profile. Based on the scree plot (ESM Fig. 1) of the oxylipins that loaded onto the PCs, we selected PC1 and PC2 to reflect distinct oxylipin profiles in DAISY children. In order to ensure that association testing of these PCs with outcomes would be comparable across critical timepoints (described in the Methods/Statistical analysis section), we applied the loadings from these two PCs to the oxylipin levels at the critical timepoints to create comparable PCs for each analysis, as described previously [23].
Relationships between oxylipins and their precursor FAs. Boxes represent oxylipins and ovals represent precursor FAs. Biosynthetic enzymes for the formation of oxylipins (CYP450 sEH, 12/15-LOX, 5-LOX, 15-LOX, COX) are indicated above the oxylipin formed by the enzyme. Full names of oxylipins are presented in ESM Table 1. COX, cyclooxygenase; DGLA, dihomo-γ-linolenic acid; GLA, γ-linolenic acid; 5-LOX, 5-lipoxygenase; 12/15-LOX, 12/15-lipoxygenase; 15-LOX, 15-lipoxygenase
Statistical analysis
We investigated the association between the 36 oxylipins (Fig. 2) and development of type 1 diabetes in 72 type 1 diabetes cases and 71 control participants. The intercepts, representing the age-adjusted mean measure of the oxylipin prior to diagnosis, were tested individually in a logistic regression model with case status as the outcome, adjusting for the high-risk genotype HLA-DR3/4,DQB1*0302. The intercepts were standardised to a mean of 0 and standard deviation of 1 so that effect sizes could be compared across the oxylipins. We used a Benjamini–Hochberg-corrected [24] p value of 0.1 to account for multiple comparisons. In addition, we tested PC1 and PC2 of the oxylipins in a logistic regression model, adjusting for HLA-DR3/4 genotype, to examine whether an oxylipin profile prior to diagnosis was associated with type 1 diabetes.
In order to investigate whether the oxylipin profile at certain points in disease progression was more indicative of risk of type 1 diabetes than the profile of mean oxylipin levels prior to diagnosis, we conducted analyses at two critical timepoints. In the nested type 1 diabetes case–control study, we selected the visit prior to IA seroconversion as the pre-SV critical timepoint. Among the 72 type 1 diabetes cases and 71 control participants, 26 type 1 diabetes cases and 31 control participants had oxylipins measured at this timepoint. We selected the visit most immediately after seroconversion as the post-SV critical timepoint, and 64 type 1 diabetes cases and 70 control participants had oxylipins measured at this timepoint. We projected the loadings of PC1 and PC2 from the above-described PCA of the 343 DAISY children onto the oxylipin levels at each critical timepoint and tested the resulting PC1 and PC2 in a logistic regression with type 1 diabetes as the outcome, adjusting for HLA-DR3/4 genotype and age at visit. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Study population
Type 1 diabetes cases were more likely to have a high-risk HLA-DR3/4 genotype than control participants (p value <0.001, Table 1). First-degree relative status, sex and ethnicity were not significantly different between type 1 diabetes cases and control participants. The mean age at type 1 diabetes diagnosis among cases was 9.7 years.
Individual mean oxylipins
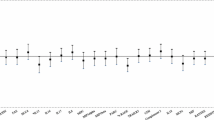
Of the 36 age-adjusted mean oxylipin levels examined prior to type 1 diabetes, nine were found to be significantly associated with type 1 diabetes using a Benjamini–Hochberg false discovery rate (FDR) significance level of α = 0.1 (Fig. 3). For 1 standard deviation increase in mean ARA-related 5-HETE levels, odds of type 1 diabetes increased by 53%. The LA-related oxylipins 13S-HODE, 9-HODE, 9-HOTE, 12(13)-EpOME and 9(10)-EpOME were inversely associated with type 1 diabetes (Fig. 3). The ALA-related oxylipins 9,19-DiHODE and α-12(13)-EpODE were inversely associated with type 1 diabetes. The DHA-related oxylipin 17-HDoHE was also inversely associated with type 1 diabetes; for every 1 standard deviation increase of mean 17-HDoHE levels, odds of type 1 diabetes decreased by 40%. No EPA-related oxylipins were associated with change in type 1 diabetes risk.
The age-adjusted means of 36 oxylipins were tested in a logistic regression model for odds of type 1 diabetes, adjusted for HLA-DR3/4 genotype. OR and 95% upper CI and lower CI for a standard deviation of 1 in mean oxylipin levels are presented. Oxylipins marked by † meet Benjamini–Hochberg FDR significance level of α=0.1. Oxylipin intercepts (age-adjusted mean) were analysed through PCA, and the first two PCs were selected. PC1 represents all LA- and ALA-related oxylipins; PC2 represents ARA-related oxylipins. PCs marked by * meet nominal significance at p value <0.05. Full names of oxylipins are presented in ESM Table 1
Profiles of mean oxylipins
Because oxylipins share precursor FAs and enzymes, and because the results from the mean oxylipin model appeared to be grouped by precursor FAs, we tested the PC1 and PC2 of the oxylipins for association with type 1 diabetes. PC1 had all LA-related and ALA-related oxylipins strongly and positively loaded and was strongly representative of higher-than-average LA- and ALA-related oxylipins (ESM Table 2). PC1 was significantly associated with a reduced risk of type 1 diabetes (OR 0.61; p value 0.02; 95% CI 0.40, 0.94). PC2 had ten of 14 ARA-related oxylipins positively loaded, as well as two DHA-related oxylipins and one EPA-related oxylipin positively loaded. PC2 was associated with an increased risk of type 1 diabetes (OR 1.53; p value 0.04; 95% CI 1.03, 2.29).
Profiles of oxylipins at critical timepoints
To investigate whether oxylipin profiles at specific points in disease progression were more indicative of odds of type 1 diabetes than a profile of mean oxylipin levels over all measured visits prior to type 1 diabetes, we examined two critical timepoints: the visit just before the case seroconverted (pre-SV) and the visit just after the case seroconverted (post-SV). Descriptions of the participants in each critical timepoint analysis are provided in ESM Table 3. We projected PCA loadings from the two PCs from the mean oxylipin PCA to the oxylipin levels at pre-SV and post-SV, so that PCs were directly comparable between the critical timepoints and the mean oxylipin analysis. Adjusting for HLA-DR3/4 genotype and age at visit, PC1 and PC2 at the pre-SV critical timepoint were not significantly associated with type 1 diabetes (Table 2). At the post-SV critical timepoint, PC2 was significantly associated with increased odds of type 1 diabetes (OR 1.79; 95% CI 1.15, 2.77).
Discussion
In children at risk for type 1 diabetes, we found that a profile of higher mean levels of LA- and ALA-related oxylipins was associated with a decreased risk of type 1 diabetes. A profile higher in ARA-related oxylipins was associated with an increased risk of type 1 diabetes. This association was observed after IA seroconversion but not before seroconversion of the type 1 diabetes cases, which may indicate the inflammation and damage in islet cells occurring during the autoimmune process.
Bone et al found that inhibiting ARA-related eicosanoids reduced islet cell damage in NOD mice through reducing TNF-α, leading to reduced T cell and B cell activity [25]. We found that the proinflammatory [26] ARA-related oxylipin 5-HETE was significantly associated with an increased risk of type 1 diabetes. 5-HETE is stimulated by oxidative stress, and promotes an inflammatory signal through G-protein-coupled receptors [26]. High levels of proinflammatory oxylipins may be indicative of uncontrolled and sustained inflammation in and damage to pancreatic islet cells.
LA-related oxylipins had a protective association with type 1 diabetes, when testing individual oxylipins and the PCs. Five of the ten measured LA-related oxylipins were significantly associated with a decreased risk of type 1 diabetes. Although LA is one of the n-6 FAs, which are generally associated with promoting inflammation, LA also plays a role in resolving inflammation [27]. LA reduces mitochondrial damage inflicted through streptozotocin (STZ) [28]. The protective effect of LA was also found by Suresh and Das: LA reduced severity of alloxan-induced diabetes, potentially through activation of peroxisome proliferator-activated receptor γ (PPARγ), which is signalled through lipid mediators [29]. Therefore, LA-related oxylipins may reduce risk of type 1 diabetes through attenuating mitochondrial damage and activating PPARγ.
The protective effects of LA in autoimmune and inflammatory conditions have been observed in population studies. The DIPP cohort demonstrated that serum LA was marginally associated with a decreased risk of IA in children at risk of type 1 diabetes [30]. A Mendelian randomisation study testing SNPs strongly associated with LA demonstrated that LA levels were protective against the autoimmune disorders rheumatoid arthritis and systemic lupus erythematosus [31]. Mendelian randomisation has also demonstrated that LA has protective effects for asthma, which is immune-mediated [32]. These studies support the protective role we found with LA-related oxylipins and type 1 diabetes.
PC1 also represented oxylipins related to ALA, another 18-carbon FA. The ALA-related oxylipins 9,10-DiHODE and α-12(13)-EpODE were significantly associated with a decreased risk of type 1 diabetes. The anti-inflammatory nature of ALA is often attributed to its conversion to EPA and DHA. However, ALA-related oxylipins have been found to have distinct pro-resolving properties through suppression of M1 macrophages [33]. These ALA-related oxylipins, as with LA-related oxylipins, may reduce the risk of type 1 diabetes through resolution of inflammation.
PC1 represented an oxylipin profile high in LA- and ALA-related oxylipins. Increased LA-related oxylipins in this study may reflect higher levels of n-3 FAs, as suggested by a study in which dietary n-3 FAs increased levels of LA-related oxylipins in the kidney in rats [34]. Similarly, ALA treatment in macrophages not only increased ALA-related oxylipins, but also increased LA-related oxylipins [33]. The close relationship between ALA- and LA-related oxylipins could explain the strong pattern found in PC1. In mice, dietary intake of LA and ALA attenuated STZ-induced diabetes, demonstrating that LA and ALA may work in tandem to reduce risk of islet injury and thereby type 1 diabetes [35]. The inverse association between this oxylipin profile and type 1 diabetes at the pre-SV critical timepoint was suggestive but not statistically significant, likely due to the small sample size in this analysis.
Although LA and ARA are both n-6 FAs, ARA-related oxylipins were associated with an increased risk of type 1 diabetes, while LA-related oxylipins were associated with a decreased risk of type 1 diabetes. The difference between LA- and ARA-related oxylipins may be related to resiliency to stressors through promotion and resolution of inflammation, which has been proposed as a model for health. Oxylipins have been used as markers of this resiliency to stress [36]. LA-related oxylipins exhibit both proinflammatory and pro-resolving effects [27]. The protective effect of LA-related oxylipins may represent a child’s ability to respond to a stressor through promotion and subsequent resolution of inflammation. ARA-related oxylipins, in contrast, do not demonstrate the same pro-resolution properties as LA-related oxylipins [10], which may explain our findings.
An increased mean level of the DHA-related oxylipin 17-HDoHE was associated with a decreased risk of type 1 diabetes. 17-HDoHE is an anti-inflammatory oxylipin that is elevated with consumption of fish oil [37], which may partially explain the beneficial effect of fish oil in type 1 diabetes. When testing oxylipins individually through the mean oxylipin approach, DHA-related oxylipins demonstrate a decreased risk of type 1 diabetes. Interestingly, the DHA-related oxylipins 19,20-DiHDPE and 14-HDoHE (which were not significant using the mean approach) also loaded positively on to PC2 in the mean oxylipin PCA, which was associated with an increased risk of type 1 diabetes. 19,20-DiHDPE is synthesised from the enzyme cytochrome P450 (CYP450) soluble epoxide hydrolase (sEH). Oxylipins derived from sEH are less active and potentially inactive [38]. The combination of n-3 FAs and an sEH inhibitor may be an effective treatment for inflammatory conditions [39]. Further, inhibition of sEH decreases lipoxin levels in severe asthma [40] and shifts macrophages to the anti-inflammatory M2 phenotype [41]. These studies demonstrate that n-3 FA oxylipins synthesised by CYP450 sEH may not share the beneficial effects of other n-3 FA oxylipins and may explain the detrimental association seen with 19,20-DiHDPE and type 1 diabetes. The association of these DHA-related oxylipins with increased risk of type 1 diabetes may also be an artefact of these oxylipins being synthesised alongside ARA-related oxylipins that increase risk of type 1 diabetes.
Strengths of this study include the longitudinal study design and information on the timing of IA seroconversion. A limitation of the study is that we did not have information on levels of biosynthetic enzymes for the oxylipins measured, which may give more information on inflammatory signals. This study was conducted in participants at increased risk of type 1 diabetes, so generalisability to other populations may be limited. Sample size at the pre-SV critical timepoint was reduced compared with the post-SV and mean oxylipin analysis, limiting the power to detect an association at the pre-SV visit. Samples were non-fasting, so recent dietary intake of FAs may have affected oxylipin levels [42] and thus added noise to the measurements.
Conclusions
This study examined longitudinal plasma oxylipin levels and risk of type 1 diabetes in children at risk of type 1 diabetes. We found that an oxylipin profile with higher levels of LA- and ALA-related oxylipins was associated with a decreased risk of type 1 diabetes. These oxylipins represent both pro-resolving and proinflammatory mediators and may be reflective of resiliency to environmental triggers. The DHA-related oxylipin 17-HDoHE was strongly protective against type 1 diabetes and provides further evidence that n-3 FAs reduce risk of type 1 diabetes. ARA-related oxylipins associated with an increased risk of type 1 diabetes may indicate inflammation following development of IA.
Data availability
The dataset used for this analysis is available upon request. No applicable resources were generated or analysed during the current study.
Abbreviations
- ALA:
-
α-Linolenic acid
- ARA:
-
Arachidonic acid
- CYP450:
-
Cytochrome P450
- DAISY:
-
Diabetes Autoimmunity Study in the Young
- DHA:
-
Docosahexaenoic acid
- DIPP:
-
Type 1 Diabetes Prediction and Prevention Study
- EPA:
-
Eicosapentaenoic acid
- FA:
-
Fatty acid
- FDR:
-
False discovery rate
- GADA:
-
GAD autoantibodies
- IA:
-
Islet autoimmunity
- IA-2:
-
Insulinoma-associated-2 autoantibodies
- IAA:
-
Insulin autoantibodies
- LA:
-
Linoleic acid
- LOQ:
-
Limit of quantification
- PC:
-
Principal component
- PCA:
-
Principal component analysis
- Post-SV:
-
Post-seroconversion
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- Pre-SV:
-
Pre-seroconversion
- sEH:
-
Soluble epoxide hydrolase
- STZ:
-
Streptozotocin
References
Bach JF (1994) Insulin-dependent diabetes mellitus as an autoimmune disease. Endocr Rev 15(4):516–542. https://doi.org/10.1210/edrv-15-4-516
Noble JA, Erlich HA (2012) Genetics of type 1 diabetes. Cold Spring Harb Perspect Med 2(1):a007732. https://doi.org/10.1101/cshperspect.a007732
Norris JM, Johnson RK, Stene LC (2020) Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol 8(3):226–238. https://doi.org/10.1016/S2213-8587(19)30412-7
Calder PC (2017) Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans 45(5):1105–1115. https://doi.org/10.1042/BST20160474
Innes JK, Calder PC (2018) Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fat Acids 132:41–48. https://doi.org/10.1016/j.plefa.2018.03.004
Norris JM, Kroehl M, Fingerlin TE, Frederiksen BN, Seifert J, Wong R et al (2014) Erythrocyte membrane docosapentaenoic acid levels are associated with islet autoimmunity: the Diabetes Autoimmunity Study in the Young. Diabetologia. 57(2):295–304. https://doi.org/10.1007/s00125-013-3106-7
Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M et al (2007) Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 298(12):1420–1428. https://doi.org/10.1001/jama.298.12.1420
Simopoulos AP (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56(8):365–379. https://doi.org/10.1016/S0753-3322(02)00253-6
Niinistö S, Takkinen HM, Erlund I, Ahonen S, Toppari J, Ilonen J et al (2017) Fatty acid status in infancy is associated with the risk of type 1 diabetes-associated autoimmunity. Diabetologia. 60(7):1223–1233. https://doi.org/10.1007/s00125-017-4280-9
Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM (2015) Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv Nutr 6(5):513–540. https://doi.org/10.3945/an.114.007732
Diab J, Al-Mahdi R, Gouveia-Figueira S, Hansen T, Jensen E, Goll R et al (2019) A Quantitative Analysis of Colonic Mucosal Oxylipins and Endocannabinoids in Treatment-Naïve and Deep Remission Ulcerative Colitis Patients and the Potential Link With Cytokine Gene Expression. Inflamm Bowel Dis 25(3):490–497. https://doi.org/10.1093/ibd/izy349
James MJ, Cleland LG (1997) Dietary n-3 fatty acids and therapy for rheumatoid arthritis. Semin Arthritis Rheum 27(2):85–97. https://doi.org/10.1016/S0049-0172(97)80009-1
Coras R, Pedersen B, Narasimhan R, Brandy A, Mateo L, Prior A et al (2020) Imbalance Between Omega-6 and Omega-3 Derived Bioactive Lipids in Arthritis in the Elderly. J Gerontol A Biol Sci Med Sci 76(3):415–425
Buckner T, Vanderlinden LA, Johnson RK et al (2020) Predictors of oxylipins in a healthy pediatric population. Pediatr Res. https://doi.org/10.1038/s41390-020-1084-2
Roep BO, Kleijwegt FS, van Halteren AG, Bonato V, Boggi U, Vendrame F et al (2010) Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clin Exp Immunol 159(3):338–343. https://doi.org/10.1111/j.1365-2249.2009.04087.x
Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA et al (2017) Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 127(4):1589. https://doi.org/10.1172/JCI92172
Cabrera SM, Henschel AM, Hessner MJ (2016) Innate inflammation in type 1 diabetes. Transl Res 167(1):214–227. https://doi.org/10.1016/j.trsl.2015.04.011
Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S et al (2019) The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur Cardiol 14(1):50–59. https://doi.org/10.15420/ecr.2018.33.1
Bonifacio E, Yu L, Williams AK, Eisenbarth GS, Bingley PJ, Marcovina SM et al (2010) Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 95(7):3360–3367. https://doi.org/10.1210/jc.2010-0293
Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P et al (2007) The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A 104(43):17040–17045
Wu J, Gouveia-Figueira S, Domellof M, Zivkovic AM, Nording ML (2016) Oxylipins, endocannabinoids, and related compounds in human milk: Levels and effects of storage conditions. Prostaglandins Other Lipid Mediat 122:28–36. https://doi.org/10.1016/j.prostaglandins.2015.11.002
Pedersen TL, Newman JW (2018) Establishing and Performing Targeted Multi-residue Analysis for Lipid Mediators and Fatty Acids in Small Clinical Plasma Samples. In: Giera M (ed) Clinical Metabolomics: Methods and Protocols. Springer New York, New York, NY, pp 175–212
Lovell AL, Davies PSW, Hill RJ, Milne T, Matsuyama M, Jiang Y et al (2019) A comparison of the effect of a Growing Up Milk - Lite (GUMLi) v. cows’ milk on longitudinal dietary patterns and nutrient intakes in children aged 12-23 months: the GUMLi randomised controlled trial. Br J Nutr 121(6):678–687. https://doi.org/10.1017/S0007114518003847
Benjamini Y, Hochberg Y (1995) Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol 57(1):289–300
Bone RN, Gai Y, Magrioti V, Kokotou MG, Ali T, Lei X et al (2015) Inhibition of Ca2+-independent phospholipase A2beta (iPLA2beta) ameliorates islet infiltration and incidence of diabetes in NOD mice. Diabetes. 64(2):541–554. https://doi.org/10.2337/db14-0097
Bittleman DB, Casale TB (1995) 5-Hydroxyeicosatetraenoic acid (HETE)-induced neutrophil transcellular migration is dependent upon enantiomeric structure. Am J Respir Cell Mol Biol 12(3):260–267. https://doi.org/10.1165/ajrcmb.12.3.7873191
Andres Contreras G, De Koster J, de Souza J, Laguna J, Mavangira V, Nelli RK et al (2019) Lipolysis modulates the biosynthesis of inflammatory lipid mediators derived from linoleic acid in adipose tissue of periparturient dairy cows. J Dairy Sci 103(2):1944–1955
Jeng JY, Yeh TS, Chiu YH, Lee YC, Cheng HH, Hsieh RH (2009) Linoleic acid promotes mitochondrial biogenesis and maintains mitochondrial structure for prevention of streptozotocin damage in RIN-m5F cells. Biosci Biotechnol Biochem 73(6):1262–1267
Suresh Y, Das UN (2003) Long-chain polyunsaturated fatty acids and chemically induced diabetes mellitus: effect of omega-6 fatty acids. Nutrition. 19(2):93–114. https://doi.org/10.1016/S0899-9007(02)00856-0
Virtanen SM, Niinisto S, Nevalainen J, Salminen I, Takkinen HM, Kaaria S et al (2010) Serum fatty acids and risk of advanced beta-cell autoimmunity: a nested case-control study among children with HLA-conferred susceptibility to type I diabetes. Eur J Clin Nutr 64(8):792–799. https://doi.org/10.1038/ejcn.2010.75
Lee YH (2018) Role of linoleic acid in autoimmune disorders: a Mendelian randomisation study. Ann Rheum Dis 78(5):711–713
Zhao JV, Schooling CM (2019) The role of linoleic acid in asthma and inflammatory markers: a Mendelian randomization study. Am J Clin Nutr 110(3):685–690. https://doi.org/10.1093/ajcn/nqz130
Pauls SD, Rodway LA, Winter T, Taylor CG, Zahradka P, Aukema HM (2018) Anti-inflammatory effects of alpha-linolenic acid in M1-like macrophages are associated with enhanced production of oxylipins from alpha-linolenic and linoleic acid. J Nutr Biochem 57:121–129. https://doi.org/10.1016/j.jnutbio.2018.03.020
Leng S, Winter T, Aukema HM (2018) Dietary ALA, EPA and DHA have distinct effects on oxylipin profiles in female and male rat kidney, liver and serum. J Nutr Biochem 57:228–237. https://doi.org/10.1016/j.jnutbio.2018.04.002
Leikin-Frenkel A, Canetti L, Halpern Z (2004) Effects of nutritional lipids on diabetic manifestations and Δ6 desaturase mRNA level in streptozotocin treated mice. Nutr Res 24:303–312. https://doi.org/10.1016/j.nutres.2003.11.006
Wopereis S, Wolvers D, van Erk M, Gribnau M, Kremer B, van Dorsten FA et al (2013) Assessment of inflammatory resilience in healthy subjects using dietary lipid and glucose challenges. BMC Med Genet 6:44
Zulyniak MA, Roke K, Gerling C, Logan SL, Spriet LL, Mutch DM (2016) Fish oil regulates blood fatty acid composition and oxylipin levels in healthy humans: A comparison of young and older men. Mol Nutr Food Res 60(3):631–641. https://doi.org/10.1002/mnfr.201500830
Capozzi ME, Hammer SS, McCollum GW, Penn JS (2016) Epoxygenated Fatty Acids Inhibit Retinal Vascular Inflammation. Sci Rep 6:39211
Swardfager W, Hennebelle M, Yu D, Hammock BD, Levitt AJ, Hashimoto K et al (2018) Metabolic/inflammatory/vascular comorbidity in psychiatric disorders; soluble epoxide hydrolase (sEH) as a possible new target. Neurosci Biobehav Rev 87:56–66. https://doi.org/10.1016/j.neubiorev.2018.01.010
Ono E, Dutile S, Kazani S, Wechsler ME, Yang J, Hammock BD et al (2014) Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. Am J Respir Crit Care Med 190(8):886–897. https://doi.org/10.1164/rccm.201403-0544OC
Wang L, Opland D, Tsai S, Luk CT, Schroer SA, Allison MB et al (2014) Pten deletion in RIP-Cre neurons protects against type 2 diabetes by activating the anti-inflammatory reflex. Nat Med 20(5):484–492. https://doi.org/10.1038/nm.3527
Rajamani A, Borkowski K, Akre S, Fernandez A, Newman JW, Simon SI et al (2019) Oxylipins in triglyceride-rich lipoproteins of dyslipidemic subjects promote endothelial inflammation following a high fat meal. Sci Rep 9(1):8655
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
The study was performed at the Barbara Davis Center for Childhood Diabetes in Denver, CO, USA. This work was supported by the National Institutes of Health R01-DK104351 and R01-DK32493. Support was also provided by NIH/NCATS Colorado CTSA Grant Number TL1 TR002533.
Author information
Authors and Affiliations
Contributions
TB designed the study, performed data analysis, interpreted the data and drafted the manuscript. JMN designed the study, interpreted the data and edited the manuscript. BCD and OF collected data and reviewed and edited the manuscript. LAV, PMC, KK, FD, BIF, MC-S and MR interpreted the data and reviewed and edited the manuscript. All authors approved the final version of the manuscript. JMN is the guarantor of this work.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM
(PDF 152 kb)
Rights and permissions
About this article
Cite this article
Buckner, T., Vanderlinden, L.A., DeFelice, B.C. et al. The oxylipin profile is associated with development of type 1 diabetes: the Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia 64, 1785–1794 (2021). https://doi.org/10.1007/s00125-021-05457-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-021-05457-9