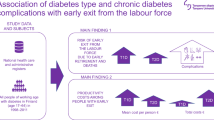
Abstract
Aims/hypothesis
The risk of complications and medical consequences of type 2 diabetes are well known. Hospital costs have been identified as a key driver of total costs in studies of the economic burden of type 2 diabetes. Less evidence has been generated on the impact of individual diabetic complications on the overall societal burden. The objective of this study was to analyse costs of hospital-based healthcare (inpatient and outpatient care) and work absence related to individual macrovascular and microvascular complications of type 2 diabetes in Sweden in 2016.
Methods
Data for 2016 were retrieved from a Swedish national retrospective observational database cross-linking individual-level data for 1997–2016. The database contained information from population-based health, social insurance and socioeconomic registers for 392,200 people with type 2 diabetes and matched control participants (5:1). Presence of type 2 diabetes and of diabetes complications were derived using all years, 1997–2016. Costs of hospital-based care and of absence from work due to diabetes complications were estimated for the year 2016. Regression analysis was used for comparison with control participants to attribute absence from work to individual complications, and to account for joint presence of complications.
Results
Use of hospital care for complications was higher in type 2 diabetes compared with control participants in 2016: 26% vs 12% had ≥1 hospital contact; there were 86,104 vs 24,608 outpatient visits per 100,000 people; and there were 9894 vs 2546 inpatient admissions per 100,000 people (all p < 0.001). The corresponding total costs of hospital-based care for complications were €919 vs €232 per person (p < 0.001), and 74.7% of costs were then directly attributed to diabetes (€687 per person). Regression analyses distributed the costs of days absent from work across diabetes complications per se, basic type 2 diabetes effect and unattributed causes. Diabetes complications amounted to €1317 per person in 2016, accounting for possible complex interactions (25% of total costs of days absent). Key drivers of costs were the macrovascular complications angina pectoris, heart failure and stroke; and the microvascular complications eye diseases, including retinopathy, kidney disease and neuropathy. Early mortality in working ages cost an additional €579 per person and medications used in risk-factor treatment amounted to €418 per person.
Conclusions/interpretation
The economic burden of complications in type 2 diabetes is substantial. Costs of absence from work in this study were found to be greater than of hospital-based care, highlighting the need for considering treatment consequences in a societal perspective in research and policy.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Type 2 diabetes is a prevalent and costly chronic disease associated with several health complications. Worldwide, the prevalence of diabetes has increased over the last decades [1] and, in 2019, there were about 463 million adults with diabetes according to the International Diabetes Federation [2]. Rising health expenditure in absolute values and as percentage of gross national product, as shown in, for instance, Organisation for Economic Cooperation and Development (OECD) health data [3], is a concern for decision makers in national health systems as well as insurance-based systems.
The economic burden of diabetes complications is known to go beyond resource use and costs in the health sector. Previous studies on the cost burden of type 2 diabetes have demonstrated that costs from reduced productivity (including e.g. work days absent, reduced labour force participation, mortality) constitute a substantial part of the total cost burden, as shown by, for instance, the most recent updates on economic costs of diabetes in the USA from 2017 and in the UK from 2012 [4,5,6]. Less evidence has been generated on the economic impact of individual diabetic complications and little is known about which complications are the key drivers of the overall societal burden. The identification of key cost drivers among complications informs research and policy of where further actions and tools are needed. Such data support clinicians, employers, organisations and society with facts on the value of preventing and postponing the incidence of diabetes complications and the extent of the economic burden falling on the individual where insurance coverage is lacking or inadequate.
Studies on the cost of illness combine data from a mix of sources, often using aggregate costs where a part of the costs is attributed to the specific disease. Complications related to type 2 diabetes are conditions that may also occur in the general population, such as cardiovascular diseases. Hence, analyses of the costs of type 2 diabetes usually report the total costs incurred by people with diabetes and the cost attributed to diabetes when applying top-down estimation strategies. For example, the ADA's report on the economic costs of diabetes in 2017 attributed 57% of the hospital inpatient care costs incurred by people with diabetes to diabetes per se (US$70 billion of US$123 billion) [4]. Costs of disease may also be derived from individual-level data with population coverage, preferably holding longitudinal information from multiple administrative registers. A Danish study of number of days absent from work due to diabetes-related complications showed that several of the investigated complications led to more than 3 months of work absence for the average person with diabetes [6].
Recent years have seen an increase in studies using diagnostic codes and administrative registers to identify people with diabetes and conduct epidemiological, clinical and economic research. Some examples include studies on total costs of care for people with diabetes [7,8,9,10], on prevalence and incidence of diabetes [11, 12] and on types of glucose-lowering medication and clinical outcomes [13]. Similar topics have also been addressed using patient cohorts registered in quality registers or incidence registers and using outcome data from national health registers in Sweden [14,15,16,17,18,19,20,21].
Sweden has a long tradition of reporting population-based information into registers, and data can be record-linked between registers for research using the Swedish personal identification number. Register-based definitions of diabetes may rely on diabetes diagnosis solely [12] or use a combination of observed healthcare contacts with diabetes diagnosis and filled prescriptions of different types of glucose-lowering medications [8, 13, 22]. This study used the latter strategy with the purpose of estimating costs of hospital (inpatient and outpatient) care and costs of absence from work related to individual macrovascular and microvascular complications of type 2 diabetes in Sweden. Using cross-linked microdata, matched control participants and straight-forward empirical strategies, this study addresses previously identified research gaps in the economic costs of type 2 diabetes and the contributions of individual diabetes complications. It also provides new insights into the balance of costs between the healthcare sector and other sectors.
Methods
Data
The analysis of the cost burden of type 2 diabetes complications in 2016 used data from a retrospective observational study database [22]. The database cross-links 20 years of individual-level data, 1997–2016, from three national authorities in Sweden: the National Board of Health and Welfare (NBHW), Försäkringskassan (the Swedish Social Insurance Agency) and Statistics Sweden. The NBHW identified people with diabetes and provided data from the National Patient Register (NPR) on outpatient and inpatient hospital healthcare [23], and from the National Prescribed Drugs Register (NPDR) on filled prescriptions of selected drugs [24]. Further register description is available in electronic supplementary material (ESM) Table 1. Demographic and socioeconomic background characteristics were obtained from Statistics Sweden from the Register of the Total Population and the LISA database (longitudinal integrated database for health insurance and labour market studies [25]). Försäkringskassan supplied data on days absent from work from the MiDAS database (Micro-Data for the Analysis of Social Insurance) covering schemes of sickness and rehabilitation benefits, and sickness and activity compensation [26]. This study calculated costs of complications in the year 2016 based on use of hospital care and absence from work in 2016. To identify the population with type 2 diabetes and presence of diabetes complications, we used the full longitudinal database (1997–2016).
People with diabetes of labour market active ages (16–70 years) were selected for the database if they, at any point during 1997–2016, fulfilled at least one of the following criteria:
-
1)
At least one healthcare visit or inpatient stay with diabetes as main or secondary diagnosis by the International Classification of Diseases version 10 (ICD 10; http://apps.who.int/classifications/icd10/browse/2016/en) codes for type 1 diabetes (E10), type 2 diabetes (E11) or unspecified diabetes (E14) in any year in the NPR from 1997 to 2016.
-
2)
At least two dispensed prescriptions of glucose-lowering medication (Anatomic Therapeutic Chemical (ATC) Classification System codes, A10) with dispense dates not more than 6 months apart in the NPDR, from 1 July 2005 to 31 December 2017.
Women treated with glucose-lowering medication with a coinciding registration of pregnancy were not included in the study population but were eligible again after 2 years. An index date was defined as the first documented occurrence from 1997 and onwards in the NPR, or the first documentation in the NPDR from 1 July 2005 and onwards, whichever occurred first.
For each person with diabetes, Statistics Sweden matched control participants from the general population (5:1) using exact year of birth, sex and region of residence in the index year. This enabled incremental analysis of costs of hospital care and absence from work for people with diabetes compared with control participants. The design allowed control participants to switch to the diabetes group at a later stage if they then fulfilled inclusion criteria for diabetes. Further information on the data sources, retrieval process and control selection can be found in the ESM (pages 2–6).
The study database includes background data (demographic and socioeconomic), exposure data (diabetes complications) and outcome data (healthcare use, filled prescriptions, mortality and days absent from work).
Empirical strategies
Selecting the type 2 diabetes population
The empirical definition of type 2 diabetes was similar to previous registry-based diabetes studies [7, 8, 13]. From the study database we omitted people with type 1 diabetes defined as having inpatient and/or outpatient care registered with type 1 diabetes (ICD E10), but no registration of type 2 diabetes (E11) or unspecified diabetes (E14), as main or supplementary diagnosis in the NPR during the study period. All other cases were included as type 2 diabetes (see the ESM for more information on selection process).
Defining diabetes complications
Complications were defined using ICD codes and procedure codes (Classification of Healthcare Interventions [Klassifikation av. vårdåtgärder (KVÅ); NBHW], which holds surgical and non-surgical interventions including diagnostic procedures in line with previous studies, as referenced in ESM Table 2). The incidence and prevalence rates of complications were identified through registrations of main diagnosis in hospital-based care. The empirical strategy was not expected to capture all early stages of complications, non-emergency needs and problems not necessarily needing assistance from medical care, but to have a comprehensive coverage of use of hospital resources.
The characteristics of each complication and its expected impact on work capacity guided the empirical strategy for identifying complications as either an event or a chronic state in the estimation of absence from work. Most complications were considered chronic; that is, after the first hospital care contact, they were assumed to remain, with potential impact on absence from work for the remainder of the working life. This work used a dichotomous definition (yes/no) of presence of each complication and thus covered the range of impact through potential progression. Four complications (severe events of hypoglycaemia and hyperglycaemia requiring hospital care, ketoacidosis and coma) were considered events as commonly used in health economic evaluations. See also ESM Linkage of diabetes complications to costs.
Chronic state complications were: eye disease and diabetic retinopathy excluding vision loss and blindness (eye disease for short); vision loss and blindness; neuropathy; peripheral vascular disease; amputation; kidney disease; end-stage renal disease (ESRD) with dialysis or kidney transplantation; ischaemic heart disease (IHD); angina pectoris; heart failure; atrial fibrillation; diabetic foot and/or ulcers; and osteoarthritis.
Myocardial infarction and stroke allowed both event- and chronic state-related consequences. For these complications, an event was defined as an inpatient care episode including potential readmission within 30 days [14, 15]. Outpatient healthcare use with main diagnosis of myocardial infarction or stroke was considered part of the chronic state of history of the respective events.
Strategy for costing hospital care resource use and medications
We used diagnosis-related group (DRG) codes and the main diagnosis to assign costs to hospital-based care, pricing all NPR healthcare contacts in 2016 using contact-specific weights from the Nordic Diagnosis-Related Group (NordDRG) nomenclature [27] and the national price for a DRG weight in 2016 [28]. The proportion attributed to diabetes was estimated as the increase in costs per 100 people compared with control participants. Incremental costs of prescription medications for preventive type 2 diabetes treatment included glucose-lowering medication, and key preventive anti-hypertensive and lipid-lowering medications were estimated by comparison with control participants, but no attribution to individual complications was attempted.
Strategy for costs of absence from work
We used information from Försäkringskassan on (short-term) sickness and rehabilitation benefits, and (long-term) sickness and activity compensation, in 2016 [29]. Net days absent from work was derived using start and stop dates for periods with sick pay and the degree of sick leave. The cost of absence from work was valued by multiplying net days with age- and sex-specific earnings including payroll taxes [30] from labour market data at Statistics Sweden. The analysis included all individuals <66 years old irrespective of labour market status in 2016.
Diagnoses recorded at Försäkringskassan are insufficient as a source for attributing spells of sick leave in 2016 to a specific complication of diabetes. Instead, costs of days absent from work were attributed to listed diabetes complications using regression analyses, including matched control participants and adjusting for level of education (see the Statistical analyses section).
Costs of excess mortality in working ages were calculated based on the human capital method [30]. Age- and sex-specific excess mortality in the diabetes group compared with the general population were multiplied by discounted age- and sex-specific net earnings, including pay roll taxes and accounting for mean work force activity up to 65 years of age.
Statistical analyses
Descriptive analyses
The descriptive analyses for diabetic and control participants report demographic and socioeconomic data as number and proportion for categorical variables and as mean and standard deviation, or median and interquartile range, for continuous variables. The univariate analysis used t test for differences between study groups since the central limit theorem guarantees near normality of sample means in large-sample studies like ours [31]. The prevalence rates of complications in 2016 were measured as the rate per 100,000 people to illustrate long-term burden after first onset of the complication, and were compared using standardised differences.
The presence of multimorbidity was analysed using cross-tabulation of number of people with diabetes and pairwise combinations of studied diabetes complications in 2016 [8]. The results are presented as a bubble diagram where the size of the bubble indicates the proportion of people with diabetes having each complication, and the lines in between complications indicate the number of people with both complications (Fig. 1).
Cost analyses
The total costs of diabetes were calculated by summarising costs for hospital-based care, medication and absence from work in 2016. Ordinary least squares regression analysis investigated the correlation between costs of days absent from work and complication indicators, see Equation (1). Regression analyses have been applied in several studies of diabetes complications [6, 8, 32, 33]; in our study, the large sample size allowed the use of simple methods as the analyses depend on sample means and variances [31].
The empirical model distributed costs of days absent from work across diabetes complications, accounting for potential multiple comorbidities at the individual level, and including education as a confounding factor. It did not include demographic or other socioeconomic characteristics as the aim was to obtain an mean value across individuals irrespective of age, sex or other basic characteristics. Equation (1) shows the empirical model, including interaction effects to explore potential differences in effects of K complications on work absence across study groups (whereby K indicates the number of complications).
for individual i. The estimated cost of work absence related to a complication, for an average individual with diabetes and the complication, consisted of four parts:
The first term is the regression constant β(Constant), which is a basic mean cost of absence from work in the estimation sample. It averages costs of days absent from work for all individuals, with and without work absence, and with and without complications etc. The second term β(Diabetes) is the basic diabetes effect, which is not attributed to any specific complication but differentiates the diabetes group from control participants, including potential differences in the distribution of other diseases not explored in this study.
Of primary interest for the analyses was the part of the costs of absence from work that was associated with complications in type 2 diabetes, which is the sum of the basic complication effect and the interaction effect β(Complication) + β(Diabetes × Complication).
Results
Descriptive analysis
The study population contained 392,200 people with type 2 diabetes alive and resident in Sweden in 2016, identified in health data registers since 1997 (index years median 2008), with 1,643,170 matched control participants. The mean age was 63 years in 2016, with 59% men and 41% women (Table 1). Panel attrition after the index year was mainly due to diabetes onset in individuals originally selected as control participants (5:1). It implied that while 51% of people with diabetes still had five control participants in 2016, 0.3% had no control participants and 7.7% had only one or two control participants. This attrition led to small differences in age and sex distribution between type 2 diabetes and control participants. There was generally a lower achieved level of education among people with diabetes compared with control participants (compulsory education only 30% vs 21%, university 22% vs 33%). These data confirm an expected difference in the distribution of socioeconomic characteristics between diabetic and control participants, motivating an inclusion of the level of education in regression analysis to account for these differences.
Table 2 shows a higher morbidity among people with type 2 diabetes. The prevalence rates for ten out of 15 complications were higher in the diabetes group based on standardised differences, even though mean values were different in all complications. The largest standardised differences were found for kidney disease, eye disease and angina pectoris.
Figure 1 shows the diabetes-related comorbidity by describing the most prevalent diabetes complications and pairwise links of the most frequent interactions. Eye disease (43%) and kidney disease (16%) had the highest prevalence, and 43,000 individuals (11%) had both of these complications. The second most common interaction (n = 24,000; 6%) was between angina pectoris (11%) and eye disease. Other frequent interactions were IHD in combination with current or previous myocardial infarction (23,300; 6%), angina pectoris (22,900; 6%) and eye disease (19,800; 5%). People with myocardial infarction also had eye disease (17,400; 4%) and angina pectoris (16,300; 4%). Heart failure (n = 20,100; 5.1%) showed the most complexity with multiple complications: more than 4000 individuals had heart failure and at least one other of six studied complications (eye disease, kidney disease, IHD, angina pectoris, myocardial infarction and atrial fibrillation). Fewer interactions were found for the major cardiovascular complications of stroke (n = 23,500; 2.3%; where 5300 also had eye disease) and myocardial infarction (n = 33,900; 8.6%; where 8300 also had kidney disease).
Costs of diabetes complications in 2016
People with type 2 diabetes and complications had high hospital care consumption and absence from work in 2016 (Table 3). The total costs of hospital-based care were €360 million, of which 74.7% (€269.3 million; €687 per person with diabetes) were attributed to type 2 diabetes from comparison with corresponding mean costs among control participants. Table 3 presents two alternative estimates of the total costs for days absent from work for people with complications. By the high-level analysis accounting for having at least one recorded complication event and state, respectively, absence from work amounted to €1744 million, of which 51% (€884 million; €2254 per person with diabetes) was attributed to complications (total of event and chronic state). The total costs of hospital-based care and of absence from work related to diabetes complications in 2016 were €1153 million (€2943 per person with diabetes).
The analysis of costs of absence from work by individual complications (Table 3; Detailed analysis—by individual complication) gave a higher estimate of the total costs of absence from work (€2080 million), but the costs attributed to the diabetes complications were lower (25%; €516.4 million, €1317 per person with diabetes). By this estimate, the total costs of hospital-based care and absence from work related to diabetes complications in 2016 were €785.7 million, corresponding to €2003 per person with diabetes. IHD conditions angina pectoris and myocardial infarction generated absence from work, while the regression analysis (Table 3) did not attribute additional days absent to remaining IHD conditions. A similar pattern was seen for the event of stroke, although the state of stroke caused additional absence from work indicating long-term effects. See ESM Table 3 for more details on estimations of costs related to absence from work.
Two additional cost items from the data were not distributed across complications but are included as points of reference. First, we observed 1100 additional deaths before age 66 years compared with general age-related mortality. The loss of working-age years was valued at €227 million from lost production (€579 per person). Second, insulin and other glucose-lowering medications cost €135 million in 2016. People with type 2 diabetes had 924,902 registered prescriptions of other risk-factor medications, including drugs used for the treatment or prevention of hypertension, dyslipidaemia, eye disease and neuropathy, costing a total of €45 million of which €29 million (65%) was a diabetes increment (ESM Table 4). The total added costs for diabetes related medications were €164 million (€418 per person).
Combining results in Tables 3 and 4 shows that common complications may induce high total costs, although cost per person is low. People with type 2 diabetes used more hospital care as a group: 86,104 vs 24,608 per 100,000 outpatient visits and 9894 vs 2546 per 100,000 inpatient admissions (both p < 0.001) (Table 4). Moreover, 26% of people with type 2 diabetes had at least one hospital contact compared with 12% for control participants (p < 0.001; ESM Table 5). More than half of these (15%) had ≥1 registration of eye disease, accounting altogether for 1372 inpatient admissions and 141,631 outpatient visits. The largest differences between type 2 diabetic and control participants were found among complications common in diabetes, such as kidney disease and diabetic foot and ulcers. Heart failure and kidney disease generated most inpatient admissions among people with type 2 diabetes. Atrial fibrillation had the highest rate among control participants (578 per 100,000 control participants) but this was still a lower level than in type 2 diabetes (847 per 100,000). Total costs of hospital-based care were estimated at €919 per person for the diabetes population and at €232 for the control group (Table 5 shows results per 100 people). Inpatient admissions related to complications were key drivers. Two complications also generated sizable outpatient costs: eye disease and ESRD (Table 5).
Discussion
This study points to substantial costs of complications in hospital care for type 2 diabetes: the incremental costs of hospital-based care, about 75% of the total costs of complications, corresponded to €687 per person with diabetes. Costs outside the health sector were even higher in a societal perspective. With two alternative specifications of the analysis, costs of workdays absent attributed to diabetes complications were estimated at €1317 or €2254 per person, respectively. By both specifications, costs of absence from work were higher than costs of hospital-based care, indicating a significant contribution to overall societal burden of complications. Production loss related to excess mortality among people with type 2 diabetes of labour market active ages added further to total costs.
Detailed analyses of costs of complications indicated that the levels of costs of hospital-based care and absence from work for macrovascular complications were €96 million and €164 million, respectively. Microvascular complications had higher costs for hospital-based care (€136 million), and costs of absence from work were nearly twice as large as those for macrovascular complications (€287 million). The data presented underline the fact that common complications may add up to high societal costs even though the cost per person is low. Two examples of this are the observed costs for eye disease and kidney disease. Data allowed for separating of severe vision loss from earlier stages of eye disease and account for dialysis or kidney transplantation in kidney disease, but do not allow for further categorising. The estimates provide an indication of the mean value of potentially heterogeneous groups. ‘Other complications’ was a mixed group where the chronic conditions diabetic foot and ulcers and osteoarthritis were key drivers of societal costs. The results point at a remaining unmet need for risk-factor treatment as diabetes complications continue to generate substantial costs of hospital-based care and absence from work.
A further observation was that the percentage of total costs of hospital-based care attributed to type 2 diabetes and the percentage of total costs of work absence attributed to diabetes complications varied between complications (Table 3). For instance, the regression analysis did not attribute any of the costs of work absence to the IHD complication, while 71% of hospital costs were attributed to type 2 diabetes. Figure 1 shows that comorbidities were common among people with IHD. Both angina pectoris and myocardial infarctions were common in people with IHD and were associated with considerable costs of work absence attributed to the complication.
Previous estimates of the economic burden of diabetes differ from this study in objectives and design but provide relevant points of reference. A British study from 2012 estimated current and future costs of type 1 and type 2 diabetes, respectively, using aggregated data and assumptions from the literature. The results showed that diabetes accounted for 10% of total health resource expenditure in 2010/2011 in the UK, where costs of production loss exceed direct healthcare costs [5]. The investigators reported total costs and did not compare them with corresponding costs in the general population. On the other hand, the diabetes increment was estimated in the US study by the ADA from 2017, which reported that people with diabetes had 2.3 times higher medical expenditures compared with people with no diabetes [4]. This study included both type 1 and type 2 diabetes and did not report costs by individual diabetes complications. A German study from 2018 explored the economic impact of diabetes complications and their interactions using nation-wide statutory health insurance data. The results showed that an increased number of complications was associated with higher total costs [7, 8]. The German study, as well as two recent studies from Sweden [9, 10], reported total healthcare costs for people with type 2 diabetes but did not account for the fact that several complications are increasingly prevalent with age in the general population. Using the estimated costs of complications among control participants, we show that the fraction attributed to diabetes varies substantially between complications (Table 3).
The presence of diabetes complications was sourced from 20 years of individual-level longitudinal NPR data capturing complications serious enough to require outpatient physician care or an inpatient admission. Compared with studies with more limited follow-up [6, 7], this study is expected to provide a more comprehensive perspective on the long-term burden of diabetes complications. The record-linking between health data and social insurance registers for people with type 2 diabetes and their matched control participants provided a foundation for analyses of absence from work related to diabetes complications, which makes results more robust as they do not rely on recall in surveys or combination of results from many different sources as, for example, in the cited US and UK studies [4, 5]. Addressing the impact of all complications at the same time implied that our results were less vulnerable to double counting, especially considering multiple comorbidities. The study design also implied that we avoided double counting of burden of diabetes complications since we used the main diagnosis in hospital care. Still, this was a conservative approach as we might underestimate the costs since diabetes complications registered as secondary diagnosis with diabetes as main diagnosis were not included. A further strength of the analysis was that it also captured the long-term consequences of complications through data from the sickness and activity compensation scheme available for people with permanent loss of work capacity.
The study has limitations. It lacks national data on use of primary care related to diabetes complications and information about reduced productivity at work (‘presenteeism’) due to diabetes complications. Furthermore, the study design did not cover all people with diabetes but only those with diabetes indication in the working-age population. However, state of complication and use of hospital-based care were captured up to age 79 years. The Swedish National Diabetes Register (NDR), with an estimated coverage of 91.5%, reported that about 363,000 individuals with type 2 diabetes were registered in 2016 [34]. This indicates that, while the study design does not include people aged 80 years and older, the study population of 392,200 individuals still represents most of all cases of type 2 diabetes in Sweden. Moreover, the incremental costs of diabetes compared with the general population diminish with age [35]. Therefore, underestimation of the costs of complications in Sweden from onset of type 2 diabetes beyond study inclusion criteria was limited.
Conclusion
This study shows that the burden of complications in type 2 diabetes is substantial, and costs due to absence from work are even greater than costs of hospital-based healthcare. This poses a challenge to society in terms of loss of work capacity, and more research and emphasis on guideline implementation are needed in the field of preventive treatment of type 2 diabetes complications.
Data availability
Subject-level data from national registers hosted by Swedish national authorities are available for research after formal evaluation of the research protocol by the Swedish Ethical Review Authority and by the respective national authorities providing data. Permission to conduct research is granted on a case-by-case basis to a limited number of named people.
Abbreviations
- DRG:
-
Diagnosis-related group
- ESRD:
-
End-stage renal disease
- ICD:
-
International Classification of Diseases
- IHD:
-
Ischaemic heart disease
- NBHW:
-
National Board of Health and Welfare
- NPDR :
-
National Prescribed Drugs Register
- NPR :
-
National Patient Register
References
NCD Risk Factor Collaboration (NCD-RisC) (2016) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387(10027):1513–1530. https://doi.org/10.1016/S0140-6736(16)00618-8
International Diabetes Federation (IDF) (2019) IDF Diabetes Atlas 9th edition. Available from http://www.diabetesatlas.org/en/. Accessed 19 Nov 2019
Organisation for Economic Co-operation and Development (OECD) (2019) OECD Health Statistics 2019. Available from http://www.oecd.org/els/health-systems/health-data.htm. Accessed 4 Dec 2019
American Diabetes Association (2018) Economic costs of diabetes in the U.S. in 2017. Diabetes Care 41(5):917–928. https://doi.org/10.2337/dci18-0007
Hex N, Bartlett C, Wright D, Taylor M, Varley D (2012) Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med 29(7):855–862. https://doi.org/10.1111/j.1464-5491.2012.03698.x
Sørensen J, Ploug UJ (2013) The cost of diabetes-related complications: registry-based analysis of days absent from work. Econ Res Int 2013:1–8. https://doi.org/10.1155/2013/618039
Kähm K, Laxy M, Schneider U, Holle R (2019) Exploring different strategies of assessing the economic impact of multiple diabetes-associated complications and their interactions: a large claims-based study in Germany. Pharmacoeconomics 37(1):63–74. https://doi.org/10.1007/s40273-018-0699-1
Kähm K, Laxy M, Schneider U, Rogowski WH, Lhachimi SK, Holle R (2018) Health care costs associated with incident complications in patients with type 2 diabetes in Germany. Diabetes Care 41(5):971–978. https://doi.org/10.2337/dc17-1763
Nathanson D, Sabale U, Eriksson JW et al (2018) Healthcare cost development in a type 2 diabetes patient population on glucose-lowering drug treatment: a nationwide observational study 2006–2014. PharmacoEcon - Open 2(4):393–402. https://doi.org/10.1007/s41669-017-0063-y
Sabale U, Bodegard J, Sundstrom J et al (2015) Healthcare utilization and costs following newly diagnosed type-2 diabetes in Sweden: a follow-up of 38,956 patients in a clinical practice setting. Prim Care Diabetes 9(5):330–337. https://doi.org/10.1016/j.pcd.2015.01.001
Jansson S, Fall K, Brus O et al (2015) Prevalence and incidence of diabetes mellitus: a nationwide population-based pharmaco-epidemiological study in Sweden. Diabet Med 32(10):1319–1328. https://doi.org/10.1111/dme.12716
Tamayo T, Brinks R, Hoyer A, Kuß OS, Rathmann W (2016) The prevalence and incidence of diabetes in Germany. Dtsch Arztebl Int 113(11):177–182. https://doi.org/10.3238/arztebl.2016.0177
Nyström T, Bodegard J, Nathanson D, Thuresson M, Norhammar A, Eriksson JW (2017) Novel oral glucose-lowering drugs are associated with lower risk of all-cause mortality, cardiovascular events and severe hypoglycaemia compared with insulin in patients with type 2 diabetes. Diabetes Obes Metab 19(6):831–841. https://doi.org/10.1111/dom.12889
Adamsson Eryd S, Svensson AM, Franzén S, Eliasson B, Nilsson PM, Gudbjörnsdottir S (2017) Risk of future microvascular and macrovascular disease in people with type 1 diabetes of very long duration: a national study with 10-year follow-up. Diabet Med 34(3):411–418. https://doi.org/10.1111/dme.13266
Kiadaliri AA, Gerdtham U-G, Nilsson P, Eliasson B, Gudbjörnsdottir S, Steen Carlsson K (2013) Towards renewed health economic simulation of type 2 diabetes: risk equations for first and second cardiovascular events from Swedish register data. PLoS One 8(5):e62650. https://doi.org/10.1371/journal.pone.0062650
Persson S, Gerdtham UG, Steen Carlsson K, Swedish Childhood Diabetes Study Group (2016) Labor market consequences of childhood onset type 1 diabetes. Econ Hum Biol 23:180–192. https://doi.org/10.1016/j.ehb.2016.09.003
Steineck I, Cederholm J, Eliasson B et al (2015) Insulin pump therapy, multiple daily injections, and cardiovascular mortality in 18,168 people with type 1 diabetes: observational study. BMJ 350:h3234. https://doi.org/10.1136/bmj.h3234
Toresson Grip E, Svensson A-M, Miftaraj M et al (2019) Real-world costs of continuous insulin pump therapy and multiple daily injections for type 1 diabetes: a population-based and propensity-matched cohort from the Swedish National Diabetes Register. Diabetes Care 42(4):545–552. https://doi.org/10.2337/dc18-1850
Persson S, Dahlquist G, Gerdtham U-G, Steen Carlsson K (2018) Why childhood-onset type 1 diabetes impacts labour market outcomes: a mediation analysis. Diabetologia 61(2):342–353. https://doi.org/10.1007/s00125-017-4472-3
Persson S, Dahlquist G, Gerdtham U-G, Steen Carlsson K (2013) Impact of childhood-onset type 1 diabetes on schooling: a population-based register study. Diabetologia 56(6):1254–1262. https://doi.org/10.1007/s00125-013-2870-8
Steen Carlsson K, Landin-Olsson M, Nystrom L et al (2010) Long-term detrimental consequences of the onset of type 1 diabetes on annual earnings—evidence from annual registry data in 1990–2005. Diabetologia 53(6):1084–1092. https://doi.org/10.1007/s00125-009-1625-z
Persson S, Johansen P, Andersson E et al (2020) Days absent from work as a result of complications associated with type 2 diabetes: evidence from 20 years of linked national registry data in Sweden. Diabetes Obes Metab 22(9):1586–1597. https://doi.org/10.1111/dom.14070
National Board of Health and Welfare (NBHW) (2019) The National Patient Register. Available from http://www.socialstyrelsen.se/en/statistics-and-data/registers/register-information/the-national-patient-register/. Accessed 4 Nov 2019
National Board of Health and Welfare (NBHW) (2019) Läkemedelsregistret [in Swedish; National Prescribed Drugs Register]. Available from http://www.socialstyrelsen.se/statistik-och-data/register/alla-register/lakemedelsregistret/. Accessed 4 Nov 2019
Statistics Sweden (2019) Longitudinal integrated database for health insurance and labour market studies (LISA). Available from http://www.scb.se/en/services/guidance-for-researchers-and-universities/vilka-mikrodata-finns/longitudinella-register/longitudinal-integrated-database-for-health-insurance-and-labour-market-studies-lisa/. Accessed 4 Nov 2019
Försäkringskassan (the Swedish Social Insurance Agency) (2019) MicroData for Analysis of Social Insurance (MiDAS) [in Swedish]. Available from http://www.forsakringskassan.se/statistik/kontakta-statistikenheten. Accessed 22 Nov 2019
National Board of Health and Welfare (NBHW) (2016) Vägledning till NordDRG—Svensk version 2016 [in Swedish: Instructions for NordDRG—Swedish version 2016]. Available from http://www.socialstyrelsen.se/utveckla-verksamhet/e-halsa/klassificering-och-koder/drg/. Accessed 22 Nov 2019
National Board of Health and Welfare (NBHW) (2019) Referensvikter för slutenvårds-och öppenvårdsgrupper, NordDRG 2016 [in Swedish; Reference weights for inpatient and outpatient groups, NordDRG 2016]. Available from http://www.socialstyrelsen.se/utveckla-verksamhet/e-halsa/klassificering-och-koder/drg/. Accessed 22 Nov 2019
Försäkringskassan (the Swedish Social Insurance Agency) (2020) Sickness compensation. Available from http://www.forsakringskassan.se/myndigheter_old/arbetsformedlingen/forsakring/!ut/p/z1/hZBBT8MwDIV_Ta-xV9SRcuskYEVCmrTDSi4oZW4arUmmJFD49xjEBQk6n_zk7z1ZDxR0oLx-s0ZnG7yeWD-p9bMs8XGzWTUo9yixrbf17V1d3ldYwuEbwH-mQVDL_j15zlBL2K66vgC0qwtAWf0AC48-gBpOwn34ozUjZYpCx55yGkJ0dJysN-QFi6RPkYWINOreTpZJlon4kLPn9au0xvdX0oCKNFDkqNfIXY45n9NNgQXO8yxMCGYi8RJcgX9ZxpAydL9JOLvuvbU7d5Cp-QR3noxh/dz/d5/L2dJQSEvUUt3QS80TmxFL1o2XzgyME1CQjFBMDhTMDgwSTlIOUVGOTJHUDU3/. Accessed 15 June 2020
Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW (2015) Methods for the economic evaluation of health care programmes. Oxford University Press, Oxford
Mihaylova B, Briggs A, O'Hagan A, Thompson S (2011) Review of statistical methods for analysing healthcare resources and costs. Health Econ 20(8):897–916. https://doi.org/10.1002/hec.1653
Gerdtham U-G, Clarke P, Hayes A, Gudbjornsdottir S (2009) Estimating the cost of diabetes mellitus-related events from inpatient admissions in Sweden using administrative hospitalization data. Pharmacoeconomics 27(1):81–90. https://doi.org/10.2165/00019053-200927010-00008
Shao H, Yang S, Fonseca V, Stoecker C, Shi L (2019) Estimating quality of life decrements due to diabetes complications in the United States: the Health Utility Index (HUI) diabetes complication equation. Pharmacoeconomics 37(7):921–929. https://doi.org/10.1007/s40273-019-00775-8
Swedish National Diabetes Register (NDR) (2017) Nationella Diabetesregistret—Årsrapport 2016 [in Swedish; Swedish National Diabetes Register—Annual Report 2016 ]. Available from http://www.ndr.nu/#/arsrapport. Accessed 5 Feb 2020
Wirehn A-B, Andersson A, Östgren C-J, Carstensen J (2008) Age-specific direct healthcare costs attributable to diabetes in a Swedish population: a register-based analysis. Diabet Med 25(6):732–737. https://doi.org/10.1111/j.1464-5491.2008.02444.x
Funding
Open access funding provided by Lund University. Funding for this study was provided by Novo Nordisk A/S, Copenhagen, Denmark to the Swedish Institute for Health Economics, Lund, Sweden.
Author information
Authors and Affiliations
Contributions
All authors confirm that they meet the International Committee of Medical Journal Editors (ICJME) uniform requirements for authorship and that they have contributed to: design and collection of data; or critical analysis and interpretation of the data; and drafting/critically revising the article. All authors share the final responsibility for the content of the manuscript and the decision to submit it for publication. Material preparation, data collection and analysis were performed by EA, SP and KSC, as was the writing of the first draft of the manuscript. KSC and JJ are joint guarantors of this work.
Corresponding author
Ethics declarations
Four authors are employees of the Swedish Institute for Health Economics (EA, SP, PL, KSC), and three authors are employees of Novo Nordisk (NH, ÅE, DT). The authors had the final responsibility for submitting the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Compliance with ethical standards
ESM
(PDF 295 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Andersson, E., Persson, S., Hallén, N. et al. Costs of diabetes complications: hospital-based care and absence from work for 392,200 people with type 2 diabetes and matched control participants in Sweden. Diabetologia 63, 2582–2594 (2020). https://doi.org/10.1007/s00125-020-05277-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-020-05277-3