Abstract
Aims/hypothesis
Recent studies have demonstrated that residual beta cells may be present in some people with long-standing type 1 diabetes, but little is known about the potential impact of this finding on alpha cell function and incretin levels. This study aimed to evaluate whether insulin microsecretion could modulate glucagon and glucagon-like peptide-1 (GLP-1) responses to a mixed meal tolerance test (MMTT).
Methods
Adults with type 1 diabetes onset after the age of 15 years (n = 29) underwent a liquid MMTT after an overnight fast. Insulin microsecretion was defined when peak C-peptide levels were >30 pmol/l using an ultrasensitive assay. Four individuals with recent-onset type 1 diabetes were included as controls. Glucagon and GLP-1 responses were analysed according to C-peptide patterns.
Results
We found comparable peak values, Δ0–max levels and AUCs of glucagon and GLP-1 responses in C-peptide-positive participants (n = 9) and C-peptide-negative participants (n = 16) with long-standing diabetes and in participants with recent-onset diabetes (n = 4). Mean glucagon levels, however, differed (p = 0.01). Mean GLP-1 responses were significantly lower according to C-peptide positivity (p < 0.001, ANOVA). Interestingly, GLP-1 levels correlated to glucagon values in C-peptide-positive participants with long-standing diabetes (Pearson’s r = 0.915, p = 0.004) and in participants with recent-onset diabetes (p < 0.001) but not in C-peptide-negative participants.
Conclusions/interpretation
The glucagon response to an MMTT in people with long-standing type 1 diabetes is not reduced by the presence of residual beta cells. The reduction of GLP-1 responses according to residual C-peptide levels suggests specific regulatory pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Glucagon is synthesised by pancreatic alpha cells and counterbalances the effects of insulin on blood glucose homeostasis. Both glucagon and insulin act in a paracrine fashion given the unique cytoarchitecture of human islets [1]. Type 1 diabetes is characterised by chronic insulin insufficiency and a dysfunctional glucagon response with a paradoxical increase after meals that may be responsible for postprandial hyperglycaemia [2] and a blunted response to hypoglycaemia. This has been attributed in part to lack of paracrine suppression by endogenous insulin [3] and inadequate insulin levels following insulin therapy. The paradoxical glucagon response to oral glucose and its suppression during i.v. glucose infusion led to the hypothesis of reduced incretin secretion in people with diabetes [4].
The long-standing belief that type 1 diabetes corresponds to the complete absence of beta cells [5] has recently been challenged. It has become apparent, particularly through the use of ultrasensitive C-peptide assays, that reduction of beta cell mass is heterogeneous, as stimulated C-peptide can still be detected decades after diabetes onset, especially in those with postpubertal diabetes onset [6, 7]. Destructive insulitis in humans has a lobular distribution; some lobules keep some insulin-containing islets several years after diabetes onset, whereas others do not [2]. Alpha cells are not unaffected by the inflammatory process. The capacity of beta cells to dedifferentiate into alpha cells may accelerate the presence of many insulin-negative islets exclusively composed of alpha cells [8]. It is not known whether the presence of residual beta cells can influence alpha cell function. In addition, incretin status according to insulin microsecretion is largely unknown.
This study sought to investigate whether the presence of functional beta cells in people with type 1 diabetes modulates glucagon and glucagon-like peptide-1 (GLP-1) responses to a mixed meal tolerance test (MMTT).
Methods
Study population
Adults with type 1 diabetes were included in the analysis after giving informed consent. Investigations were carried out in accordance with the principles of the Declaration of Helsinki as revised in 2008. The entry criterion was diabetes onset after the age of 15 years. Participants (n = 29, 38% female, 100% white) had a median age of 33 years (interquartile range [IQR] 28.5–38.5 years) and a median age at diabetes onset of 24 years (IQR 19–26.5 years). After an overnight fast of at least 10 h, participants underwent a 2 h MMTT under conditions of pre-test plasma glucose between 4 and 12 mmol/l. Participants treated with an insulin pump were kept on their basal rate of insulin and those on injections had their basal insulin the night before the test. A standard liquid test of 4.4 g/100 ml fat, 17.6 g/100 ml carbohydrate and 10 g/100 ml protein (Delical HP-HC; Lactalis Nutrition Santé, Torcé, France) was given at a dose of 4 ml/kg i.e. 25.10 kJ/kg (6 kcal/kg) to a maximum of 360 ml taken in less than 2 min. Blood samples were drawn for measurements of plasma glucose, C-peptide, glucagon and GLP-1 before and 15, 30, 45, 60, 90 and 120 min after the test.
Laboratory measurements
C-peptide was measured by ultrasensitive ELISA (Mercodia, Uppsala, Sweden) with a lower detection limit of 2.5 pmol/l. Intra-assay CVs were 6.2% and 4.6% and inter-assay CVs were 4.3% and 3.5% at 15 pmol/l and 54 pmol/l, respectively. Glucagon was measured by a solid phase two-site enzyme immunoassay (Mercodia) with a detection limit of 1 pmol/l. Glucagon ELISA intra-assay CVs were 5.1% and 3.3% and inter-assay CVs were 8.1% and 7.3% at 3 pmol/l and 21.9 pmol/l, respectively. GLP-1 was measured by a total GLP-1 ELISA kit (Epitope Diagnostics, San Diego, CA, USA) with a detection limit of 0.6 pmol/l. Intra-assay CVs were 3.7% and 4.7% at 3.0 pmol/l and 10.2 pmol/l, respectively. Inter-assay CVs were 6.2% and 9.5% at 4.2 pmol/l and 12.6 pmol/l, respectively.
Statistical analysis
Data are presented as median and IQR or mean ± SEM where appropriate. The sample size was determined to establish a reduction of 40% in peak glucagon levels during the MMTT, assuming an SD of 5 pmol/l, an alpha risk of 5% with 80% power, and a two-sided t test at a 5% significance level. The required sample size was calculated to be 20 (ten participants per arm), using the package epiR, version 0.9-87 (R Foundation for Statistical Computing, Vienna, Austria). Analysis was performed using Student’s t test or the Mann–Whitney U test where appropriate. Mean values from the three groups were compared by one-way ANOVA. AUCs were calculated using GraphPad Prism software (version 7.0, www.graphpad.com). Comparisons were performed using the two-sided Wilcoxon signed-rank test. A two-tailed p value <0.05 was considered statistically significant.
Results
The mean (min–max) C-peptide response to the MMTT in 16 participants with undetectable fasting C-peptide levels was 2.52 (2.5–2.83) pmol/l. These participants were considered to be C-peptide negative. Nine participants with more than 5 years of diabetes had detectable fasting C-peptide levels and stimulated levels above 30 pmol/l. These participants were considered to be C-peptide positive. All participants with recent-onset type 1 diabetes of less than 4 years (n = 4) had mean (min–max) C-peptide levels of 234.5 (68.6–600) pmol/l. Characteristics of the participants are summarised in Table 1.
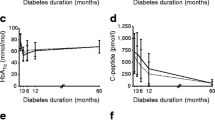
Fasting plasma glucose concentrations and AUC0–120min plasma glucose values during the MMTT were comparable between groups (Table 1). C-peptide-negative, C-peptide-positive and recent-onset diabetic participants showed comparable Δ0-max glucagon responses to the MMTT (12.31 ± 1.6, 12.09 ± 2.41 and 18.76 ± 6.19 pmol/l, respectively; p = 0.29) and identical AUCs (75.2 ± 7.44, 80.07 ± 1.5, 81.41 ± 29 pmol/l, respectively; p = 0.92), but mean values were significantly different (p < 0.02). As shown in Fig. 1a–d, glucagon and GLP-1 responses in C-peptide-positive compared with C-peptide-negative participants were delayed (Wilcoxon signed-rank test, p = 0.01), with maximum levels found for both hormones at 45 min. Mean glucagon and GLP-1 time values were correlated in C-peptide-positive participants (Pearson’s r = 0.915; p = 0.004) as well as in participants with recent-onset diabetes (Pearson’s r = 0.95; p = 0.001). By contrast, in C-peptide-negative participants (Pearson’s r = 0.214; p = 0.662) peak GLP-1 levels occurred 15 min after the MMTT, which preceded peak glucagon levels at 30 min. GLP-1 levels were significantly increased after the MMTT and mean values were significantly reduced according to C-peptide levels (p < 0.002) (Fig. 1).
Time courses of plasma blood glucose (a), C-peptide (b), GLP-1 (c) and glucagon (d) during an MMTT in participants with recent-onset type 1 diabetes (white squares, n = 4) and in those with long-standing type 1 diabetes who were either C-peptide positive (white circles, n = 9) or C-peptide negative (black circles, n = 16)
Discussion
We report a study investigating the effect of an MMTT in individuals with type 1 diabetes according to the presence or absence of residual C-peptide. Our findings showed: (1) the persistent stimulation of glucagon secretion after an oral challenge in all participants with type 1 diabetes; (2) no influence of residual beta cell function on peak glucagon levels; (3) a significant correlation between GLP-1 and glucagon secretion in C-peptide-positive individuals; (4) an inverse correlation between GLP-1 and C-peptide levels.
The demonstration that some people with long-standing type 1 diabetes have residual beta cells in their pancreas has generated many questions and hope for new treatment strategies. Several studies have identified factors associated with distinct pathogenic processes, such as age at onset or genetic background, that conditioned the number of insulin-containing islets at onset [2, 7, 8]. People with late onset diabetes have an increased chance of remaining insulin microsecretors. In this study, we found that 36% of participants with clinical diabetes onset after 15 years of age had residual beta cell function. This situation was not associated with a difference in HbA1c levels. Glucagon secretion is increased during type 1 diabetes and is not influenced by euglycaemia [9]. Surprisingly, we were not able to demonstrate a reduction in glucagon response to the MMTT that was even higher in participants with recent-onset diabetes, probably due to the amino acid composition of the liquid test. This indicates that the glucagon response cannot be reduced by significant amounts of circulating C-peptide. In addition, the remaining intact beta cells may be unable to exert paracrine interactions with the numerous alpha cells generally observed in the pancreas of people with type 1 diabetes in the context of metabolic stress. We found that GLP-1 responses were inversely correlated to C-peptide levels and that C-peptide-positive and recent-onset diabetic participants showed a significant correlation between GLP-1 and glucagon levels, in contrast with C-peptide-negative participants. A feedback loop of intestinal L cells by insulin and/or C-peptide is unlikely, since insulin was found to stimulate GLP-1 secretion. An alternative explanation for increased GLP-1 secretion in C-peptide-negative participants could be linked to reduced nutrient absorption related to pancreatic exocrine deficiency and increased delivery of nutrients and/or fermentation products to distal intestinal L cells. Aberrant expression of GLP-1 by alpha cells as shown in islets from type 2 diabetic individuals remains speculative [10]. The absence of evaluation of glucose-dependent insulinotropic polypeptide response in our study is also a limitation in understanding the role of the incretin system in type 1 diabetes. The reduction of HbA1c from baseline in type 1 diabetic individuals by the addition of liraglutide to insulin during the Efficacy and Safety of Liraglutide as Adjunct Therapy to Insulin in the Treatment of Type 1 Diabetes (ADJUNCT ONE) trial [11] was greater in C-peptide-positive individuals with fewer hypoglycaemic episodes. The capacity to enhance intra-islet GLP-1 effects in diabetic individuals with residual beta cells is an interesting issue. In an earlier study, i.v. infusion of GLP-1 in people with type 1 diabetes and residual beta cell function reduced glucagon levels and postprandial glucose excursions [12].
In conclusion, although some diabetic individuals continue to have residual beta cell function there is no evidence for changes in glucagon secretion. The capacity of alpha cells to release GLP-1 needs to be validated using pancreatic tissue from donors with type 1 diabetes. This will confirm the value of incretins as adjuvant tools in type 1 diabetes.
Data availability
All data are available from the corresponding author on request.
Abbreviations
- GLP-1:
-
Glucagon-like peptide-1
- MMTT:
-
Mixed meal tolerance test
References
Bosco D, Armanet M, Morel P et al (2010) Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes 59(5):1202–1210. https://doi.org/10.2337/db09-1177
Atkinson MA, von Herrath M, Powers AC, Clare-Salzler M (2015) Current concepts on the pathogenesis of type 1 diabetes—considerations for attempts to prevent and reverse the disease. Diabetes Care 38(6):979–988. https://doi.org/10.2337/dc15-0144
Brown RJ, Sinaii N, Rother KI (2008) Too much glucagon, too little insulin: time course of pancreatic islet dysfunction in new-onset type 1 diabetes. Diabetes Care 31(7):1403–1404. https://doi.org/10.2337/dc08-0575
Hare KJ, Vilsbøll T, Holst JJ, Knop FK (2010) Inappropriate glucagon response after oral compared with isoglycemic intravenous glucose administration in patients with type 1 diabetes. Am J Physiol Endocrinol Metab 298(4):E832–E837. https://doi.org/10.1152/ajpendo.00700.2009
Eisenbarth GS (1986) Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med 314(21):1360–1368. https://doi.org/10.1056/NEJM198605223142106
Oram RA, Jones AG, Besser RE et al (2014) The majority of patients with long duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia 57(1):187–191. https://doi.org/10.1007/s00125-013-3067-x
Wang L, Lovejoy NF, Faustman DL (2012) Persistence of prolonged C-peptide production in type 1 diabetes as measured with an ultrasensitive C-peptide assay. Diabetes Care 35(3):465–470. https://doi.org/10.2337/dc11-1236
Talchai C, Xuan S, Lin HV, Sussel L, Accili D (2012) Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150(6):1223–1234. https://doi.org/10.1016/j.cell.2012.07.029
Kramer CK, Borgoño CA, Van Nostrand P, Retnakaran R, Zinman B (2014) Glucagon response to oral glucose challenge in type 1 diabetes: lack of impact of euglycemia. Diabetes Care 37(4):1076–1082. https://doi.org/10.2337/dc13-2339
Marchetti P, Lupi R, Bugliani M et al (2012) A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia 55(12):3262–3272. https://doi.org/10.1007/s00125-012-2716-9
Mathieu C, Zinman B, Hemmingsson JU et al (2016) Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: the ADJUNCT ONE treat-to-target randomized trial. Diabetes Care 39(10):1702–1710. https://doi.org/10.2337/dc16-0691
Dupre J, Behme MT, Hramiak IM et al (1995) Glucagon-like peptide I reduces postprandial glycemic excursions in IDDM. Diabetes 44(6):626–630. https://doi.org/10.2337/diab.44.6.626
Acknowledgements
We are indebted to S. Reffet, M. Buchy and L. Kepenekian from the Department of Endocrinology and Diabetes, Lyon-Sud Hospital for their help with selecting study participants.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
CT and LM contributed to the original research idea. KC carried out the hormonal evaluations. CT was responsible for the statistical analysis and as such is the guarantor of this work and takes responsibility for the integrity of the data. All authors wrote, revised and edited the manuscript, had full access to the data, and read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
The authors declare that there is no duality of interest associated with this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thivolet, C., Marchand, L. & Chikh, K. Inappropriate glucagon and GLP-1 secretion in individuals with long-standing type 1 diabetes: effects of residual C-peptide. Diabetologia 62, 593–597 (2019). https://doi.org/10.1007/s00125-018-4804-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-018-4804-y