Abstract
Aims
Glucose-dependent insulinotropic polypeptide (GIP) is released primarily from the proximal small intestine and glucagon-like peptide-1 (GLP-1) from the more distal small intestine and colon. Their relative importance to the incretin effect in health has been contentious in the past, although it now appears that GIP has the dominant role. It is uncertain whether there is a relationship between GIP and GLP-1 secretion. We aimed to evaluate the relationship between plasma GIP and GLP-1 responses to a 75-g oral glucose load in individuals with normal (NGT) and impaired glucose tolerance (IGT).
Methods
One hundred healthy subjects had measurements of blood glucose, serum insulin, plasma GIP and GLP-1 concentrations for 240 min after a 300 mL drink containing 75 g glucose.
Results
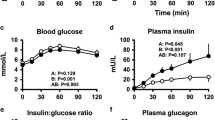
Fifty had NGT and 41 IGT; 9 had type 2 diabetes and were excluded from analysis. In both groups, there were increases in plasma GIP and GLP-1 following the glucose drink, with no difference in the magnitude of the responses between t = 0–240 min. There was a weak relationship between the iAUC0–240 min for GIP and GLP-1 in the combined (r = 0.23, P = 0.015) and in the IGT (r = 0.34, P = 0.01), but not in the NGT (r = 0.15, P = 0.14) group.
Conclusions
There is a weak relationship between oral glucose-induced GIP and GLP-1 secretions in non-diabetic subjects.


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
Drucker DJ, Nauck MA (2006) The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368(9548):1696–1705. https://doi.org/10.1016/S0140-6736(06)69705-5
Gasbjerg LS, Helsted MM, Hartmann B et al (2019) Separate and combined glucometabolic effects of endogenous glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 in healthy individuals. Diabetes 68(5):906–917. https://doi.org/10.2337/db18-1123
Nauck M, Stockmann F, Ebert R, Creutzfeldt W (1986) Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 29(1):46–52
Briere DA, Bueno AB, Gunn EJ, Michael MD, Sloop KW (2018) Mechanisms to elevate endogenous GLP-1 beyond injectable GLP-1 analogs and metabolic surgery. Diabetes 67(2):309–320. https://doi.org/10.2337/db17-0607
Madsbad S, Holst JJ (2014) GLP-1 as a mediator in the remission of type 2 diabetes after gastric bypass and sleeve gastrectomy surgery. Diabetes 63(10):3172–3174. https://doi.org/10.2337/db14-0935
Gasbjerg LS, Christensen MB, Hartmann B et al (2018) GIP(3–30)NH2 is an efficacious GIP receptor antagonist in humans: a randomised, double-blinded, placebo-controlled, crossover study. Diabetologia 61(2):413–423. https://doi.org/10.1007/s00125-017-4447-4
Frias JP, Nauck MA, Van J et al (2018) Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 392(10160):2180–2193. https://doi.org/10.1016/s0140-6736(18)32260-8
Nauck MA, El-Ouaghlidi A, Gabrys B et al (2004) Secretion of incretin hormones (GIP and GLP-1) and incretin effect after oral glucose in first-degree relatives of patients with type 2 diabetes. Regul Pept 122(3):209–217. https://doi.org/10.1016/j.regpep.2004.06.020
Nauck MA, Meier JJ (2018) Incretin hormones: Their role in health and disease. Diabetes Obes Metab 20(Suppl 1):5–21. https://doi.org/10.1111/dom.13129
Gentilcore D, Doran S, Meyer JH, Horowitz M, Jones KL (2006) Effects of intraduodenal glucose concentration on blood pressure and heart rate in healthy older subjects. Dig Dis Sci 51(4):652–656
Chan JCN, Wong RYM, Cheung C-K et al (1997) Accuracy, precision and user-acceptability of self blood glucose monitoring machines. Diabetes Res Clin Pract 36(2):91–104. https://doi.org/10.1016/S0168-8227(97)00036-3
Weitgasser R, Straberger A, Schnoll F, Sailer S (1994) Clinical evaluation of the blood glucose monitors Accutrend, Companion 2, Glucometer 3 and One Touch II in comparison with the glucose oxidase reference method. Wien Klin Wochenschr 106(23):738–741
Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15(7):539–553. https://doi.org/10.1002/(SICI)1096-9136(199807)15:7%3C539::AID-DIA668%3E3.0.CO;2-S
Trahair LG, Horowitz M, Marathe CS et al (2014) Impact of gastric emptying to the glycemic and insulinemic responses to a 75-g oral glucose load in older subjects with normal and impaired glucose tolerance. Physiol Rep 2(11):e12204. https://doi.org/10.14814/phy2.12204
Wishart J, Morris HA, Horowitz M (1992) Radioimmunoassay of gastric inhibitory polypeptide in plasma. Clin Chem 38(10):2156–2157
Trahair LG, Horowitz M, Rayner CK et al (2012) Comparative effects of variations in duodenal glucose load on glycemic, insulinemic, and incretin responses in healthy young and older subjects. J Clin Endocrinol Metabolism 97(3):844–851
Mortensen K, Petersen LL, Orskov C (2000) Colocalization of GLP-1 and GIP in human and porcine intestine. Ann N Y Acad Sci 921:469–472
Brubaker PL (1991) Regulation of intestinal proglucagon-derived peptide secretion by intestinal regulatory peptides. Endocrinology 128(6):3175–3182. https://doi.org/10.1210/endo-128-6-3175
Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W (1993) Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 91(1):301–307. https://doi.org/10.1172/jci116186
Marathe CS, Rayner CK, Bound M et al (2014) Small intestinal glucose exposure determines the magnitude of the incretin effect in health and type 2 diabetes. Diabetes 63(8):2668–2675. https://doi.org/10.2337/db13-1757
Acknowledgements
This research received no specific Grant from any funding agency.
Author information
Authors and Affiliations
Contributions
CSM conducted research, analyzed and interpreted data and reviewed paper; HP conducted research, analyzed and interpreted data and reviewed paper; JAM analyzed and interpreted data and reviewed paper; LGT conducted research and reviewed paper; LH analyzed data and reviewed paper; TW interpreted data and reviewed paper; LKP interpreted data and reviewed paper; CKR interpreted data and reviewed paper; MAN conceived and designed research and reviewed paper; MH conceived and designed research, interpreted data and reviewed paper; KLJ conceived and designed research, interpreted data, reviewed paper and approved the final version of paper.
Corresponding author
Ethics declarations
Conflict of interest
CSM is supported by a NHMRC Early Career Fellowship, TW by a Royal Adelaide Hospital Florey Fellowship and KLJ by the University of Adelaide William T Southcott Research Fellowship.
Ethical standard statement
All procedures involving human participants were in accordance with the ethical standards of the institutional research committee (Human Research Ethics Committee of the Royal Adelaide Hospital) and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marathe, C.S., Pham, H., Marathe, J.A. et al. The relationship between plasma GIP and GLP-1 levels in individuals with normal and impaired glucose tolerance. Acta Diabetol 57, 583–587 (2020). https://doi.org/10.1007/s00592-019-01461-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-019-01461-z