Abstract
Aims/hypothesis
B cell CLL/lymphoma 2 (BCL-2)-interacting cell death suppressor (BIS), known as an anti-stress and anti-apoptotic protein, has been reported to modulate susceptibility to oxidative stress. This study investigated the potential role of BIS as an antioxidant protein in diabetic nephropathy.
Methods
Diabetes was induced in BIS-heterozygote (BIS-HT) mice via streptozotocin injections and the resulting phenotypes were compared with those of BIS-wild-type (BIS-WT) mice over the 20 weeks following diabetes induction.
Results
Renal injuries, represented by increased plasma creatinine levels and increased albuminuria, were greater in diabetic BIS-HT mice than in diabetic BIS-WT mice, and were accompanied by a significant increase in reactive oxygen species (ROS) and oxidative stress markers. Moreover, renal pathological changes and the apoptotic process were accelerated in diabetic BIS-HT mice compared with diabetic BIS-WT mice with the same degree of hyperglycaemia; all were restored by 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (tempol) treatment. The levels of NADPH oxidase and related proteins were not significantly higher in diabetic BIS-HT mice compared with diabetic BIS-WT mice. However, levels of superoxide dismutase (SOD)1 and SOD2 increased on the induction of diabetes in BIS-WT mice but not in BIS-HT mice, correlating with the total SOD activity. An in vitro study showed that knockdown of BIS production also resulted in impaired induction of SOD activity as well as SOD levels in HK-2 and NMS cells, concomitant with significant ROS accumulation.
Conclusion/interpretation
Our results suggest that the decreased antioxidant capacity of BIS aggravates diabetic nephropathy in diabetic BIS-HT mice, possibly as a result of the disruption in the regulation of SOD protein quality under oxidative stress.
Similar content being viewed by others
Introduction
Diabetic nephropathy is a severe microvascular complication observed in both type 1 and type 2 diabetes; it is the leading cause of renal failure. Increasing evidence indicates that excessive reactive oxygen species (ROS) induced by chronic hyperglycaemia play a central role in the development of diabetic complications [1–3]. ROS can activate several pro-inflammatory transcriptional factors, resulting in the production of cytokines, chemokines and adhesion molecules, as well as an influx of inflammatory cells into the kidney [4, 5]. The importance of redox balance in diabetic nephropathy has been demonstrated in diabetic mice overexpressing antioxidant enzymes; these animals are protected from interstitial fibrosis and their proximal tubule cells do not undergo apoptosis [6–9]. Therefore, the preservation of antioxidant defence capacity in kidneys may be a major determinant of the severity of diabetic nephropathy caused by sustained hyperglycaemic oxidative stress.
B cell CLL/lymphoma 2 (BCL-2)-interacting cell death suppressor (BIS; also known as BCL2-associated athanogene [BAG]3 or carboxyamido-triazole-stressed [CAIR]-1), was originally identified based on its ability to interact with the BCL-2 protein and enhance its anti-apoptotic activity [10]. Recent studies have shown that BIS is involved in the regulation of cell survival in various cellular environments [11–14], as well as in protein quality during ageing [15, 16]. BIS upregulation has been observed in cells exposed to a variety of stresses [17–20]. Notably, oxidative stress has been shown to be a potent inducer of BIS expression in mouse retina or reactive astrocytes in the rat hippocampus [18, 20]. Furthermore, the suppression of BIS expression has been reported to sensitise leukaemic cells to death induced by a glutathione-depriving agent, and the overexpression of BIS attenuates apoptosis induced by the electrophilic 4-hydroxynonenal, suggesting that the induction of BIS could be related to protecting cells exposed to oxidative stress [11, 21]. However, the molecular pathway by which BIS modulates cell survival is not well understood, though the binding of BIS to BCL-2 or heat shock 70 kDa protein (HSP70) has provided some insights into this process [10, 22]. We recently reported that the downregulation of BIS in C6 glial cells leads to accelerated cell death on oxygen–glucose deprivation (OGD), accompanied by the accumulation of ROS and an impairment in the activation of superoxide dismutase (SOD)1 at the transcriptional level [23]. These findings suggest that modulation of BIS expression may affect the development of diabetic nephropathy, in which the accumulation of ROS could trigger a cascade resulting in cell death.
We previously demonstrated that the deletion of the Bis gene (also known as Bag3) in mice results in growth retardation and early lethality with serious metabolic deterioration [24, 25], suggesting that BIS is critical for postnatal growth and survival. In contrast to Bis-knockout mice, the growth and reproduction of Bis-heterozygote ([Bis +/−]; BIS-HT) mice appeared to be normal. Therefore, BIS-HT mice were used in this study to investigate whether BIS haploinsufficiency could have an effect on renal damage in a streptozotocin (STZ)-induced diabetes mouse model. Our results indicate that diabetic nephropathy is intensified in BIS-HT mice, and is accompanied by increased oxidative stress. The modulation of SOD expression and activity may be a possible molecular basis for increased susceptibility to oxidative stress in BIS-HT mice.
Methods
Experimental mouse model
The heterozygous mutant BIS-HT mice were prepared as previously described [24] and maintained on a C57/BL6 background to generate BIS-HT and Bis-wild-type (BIS-WT) littermates. Only male BIS-HT and BIS-WT mice were used for the studies. Mice were housed in a sterile environment with 12 h/12 h light/dark cycles and had free access to food and water. The induction of diabetes mellitus was performed by intraperitoneal injection of STZ (50 mg kg−1 day−1) for 5 days to BIS-HT mice of 8 weeks of age (BIS-HT DM; n = 8) and BIS-WT mice of equivalent age (BIS-WT DM; n = 8). The control mice (BIS-WT and BIS-HT) were each injected with 0.1 mmol/l sodium citrate (n = 6). To define the role of SOD in diabetic nephropathy, 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (tempol; Sigma-Aldrich, Steinheim, Germany), a SOD mimetic, was given to BIS-WT DM and BIS-HT DM mice in drinking water (20 μg kg−1 day−1) from 13 weeks after injection of STZ to 20 weeks (n = 8).
All research procedures involving animals were performed in accordance with the Laboratory Animals Welfare Act and the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) at the College of Medicine, Catholic University of Korea, Korea.
At 20 weeks after induction of diabetes, the animals were housed in metabolism cages (Nalgene, Rochester, NY, USA) for 24 h to collect urine. The mice were then killed and the kidneys were rapidly dissected and stored in buffered formalin (10%) for subsequent immunohistochemical analysis. Clinical variables in plasma and urine were determined as previously described [26].
Histology
Kidney samples were fixed in 10% (vol./vol.) buffered formalin and embedded in paraffin. Histology was assessed by haematoxylin–eosin, periodic acid–Schiff’s reagent (PAS) and Masson’s trichrome staining. Mesangial matrix area and glomerular tuft area were quantified for each glomerular cross-section using PAS-staining sections. More than 30 glomeruli that were cut through the vascular pole were counted per kidney and the average was used to determine the fractional mesangial area. We also performed immunohistochemistry for nitrotyrosine, TGF-β1, type IV collagen (Col IV), hypoxia-inducible factor 1-α (HIF-1α) and cell surface glycoprotein F4/80 (F4/80). The proportion of apoptotic cells was determined by active caspase-3 expression and TUNEL assay. See the electronic supplementary material (ESM) methods for further details.
Assessment of renal oxidative stress
To evaluate oxidative DNA damage and lipid peroxidation, we measured serum and 24 h urinary 8-hydroxy-deoxyguanosine (8-OH-dG; OXIS Health Products, Portland, OR, USA) and 24 h urinary 8-epi-prostaglandin F2α (isoprostane; OXIS Health Products) concentrations, respectively. The total SOD activities were measured using a commercial kit (Enzo Life Science, Farmingdale, NY, USA), based on the inhibition of a water-soluble tetrazolium salt-1 reduction. Determination of ROS accumulation in renal tissues and cultured cells was carried out using the oxidative fluorescent dyes dihydroethidine (DHE) and 2′7′-dichlorofluorescein diacetate (DCF-DA), respectively. For detailed information, see ESM Methods.
Cell culture and small interfering RNA transfection
Rat normal mesangial cells (NMS) cells and human kidney-2 cells (HK-2) were maintained in RPMI 1640 medium with 11.1 mmol/l of glucose and DMEM with 25 mmol/l glucose, respectively, supplemented with heat-inactivated 10% (vol./vol.) FBS (HyClone Laboratories, Logan, UT, USA). Suppression of BIS production was performed by transfection of Bis-targeted small interfering (si)RNA (50 nmol/l, 5′-AAGGUUCAGACCAUCUUGGAA-3′) using G-fectin (Genolution Pharmaceuticals, Seoul, Korea) in HK-2 cells, or Neon Transfection system (Invitrogen, Carlsbad, CA, USA) in NMS cells at 1350 V with 30 ms pulse. After 48 h of transfection, NMS and HK-2 cells were exposed to 25 and 50 mmol/l glucose (respective final concentrations based on the glucose concentration of normal culture conditions), for an additional 72 h.
Western assay and quantitative real-time PCR
Western assays were performed on total protein from the kidney tissues from each group and from in vitro cell lines using specific antibodies for NADPH oxidase (NOX)-1, NOX-4, cytochrome b-245 light chain (p22phox), Ras-related C3 botulinum toxin substrate 1 (RAC1), SOD1, SOD2 and β-actin. The transcript levels of Sod1 and Sod2 were evaluated with quantitative real-time PCR methods [27]. For the detailed procedure, see the ESM Methods and ESM Table 1.
Statistical analysis
Data were expressed as means ± SE. Differences between groups were examined by two-way ANOVA with Bonferroni’s correction (SPSS v. 11.5; IBM, Armonk, NY, USA). A p value of less than 0.05 was considered significant.
Results
Decreased renal function in diabetic BIS-HT mice
As shown in Table 1, the body weights of BIS-WT DM and BIS-HT DM mice were significantly lower than those of control BIS-WT and BIS-HT mice (p < 0.05) at 20 weeks after the induction of diabetes. The kidney-to-body-weight ratios were significantly higher in BIS-WT DM and BIS-HT DM mice compared with control BIS-WT and BIS-HT mice (p < 0.05 and p < 0.01, respectively). While there were no differences in serum lipid levels among all experimental groups, blood glucose and HbA1c levels were higher in both groups of diabetic mice compared with the controls (Table 1). However, the values of these variables were not significantly different between BIS-HT DM mice and BIS-WT DM mice. In contrast, the plasma creatinine levels were higher and creatinine clearance was lower in BIS-HT DM mice than in any other group (Fig. 1a, b). Urinary albumin excretion increased in both BIS-WT DM mice and BIS-HT DM mice, but BIS-HT DM mice exhibited more severe albuminuria than BIS-WT DM mice over the course of 20 weeks after the induction of diabetes (Fig. 1c). Thus, BIS deficiency appeared to aggravate renal function in the diabetic mouse model.
Increased renal damage in BIS-HT DM mice. Serum creatinine (a) and creatinine clearance (b) were measured in vehicle-treated control and diabetic BIS-WT (white) and BIS-HT (black) mice with or without tempol at 20 weeks following the induction of diabetes. The urinary excretion of albumin over 24 h was determined every 4 weeks up to 20 weeks in control (circles) and diabetic (squares) BIS-WT (white) and BIS-HT (black) mice (c), and the effect of tempol on albuminuria was determined at 20 weeks after diabetes induction (d). The values represent the mean ± SE of 6–8 mice/group. *p < 0.05 and ***p < 0.001 vs corresponding BIS-WT. ††† p < 0.001 vs diabetic BIS-HT mice without tempol treatment. DM, diabetes; temp, tempol
Increased oxidative stress is associated with the aggravation of diabetic nephropathy in BIS-HT mice
We then investigated whether the kidneys of BIS-HT mice were more susceptible to renal damage resulting from increased diabetes-associated oxidative stress than kidneys from BIS-WT mice. During the entire study period, the 24 h urinary isoprostane levels in BIS-HT DM mice were remarkably higher compared with the corresponding values for BIS-WT DM mice (Fig. 2a, b). Notably, after 12 weeks of diabetes, the urinary isoprostane levels of BIS-HT DM mice significantly increased and were 7.6-fold higher than those of BIS-WT DM mice 20 weeks after induction of diabetes, showing a pattern similar to the 24 h urinary albumin levels (Figs 1c, 2a). Serum 8-OH-dG levels were also significantly higher in BIS-HT DM mice than in any other mice, including BIS-WT DM mice (Fig. 2c). The 24 h urinary 8-OH-dG levels of BIS-HT DM mice were twofold higher than those of BIS-WT DM mice at 20 weeks after induction of diabetes (Fig. 2d). Furthermore, the DHE fluorescence signals, reflecting superoxide levels, were obviously increased in kidney from BIS-HT DM mice (Fig. 3a, c). In addition, staining for nitrotyrosine indicated that peroxynitrite levels, formed by nitric oxide and superoxide by diffusion-controlled reaction [28], were also increased considerably in BIS-HT DM mice (Fig. 3b, d). Thus, in addition to the oxidative stress markers in serum and urine, renal ROS levels were markedly enhanced per se in BIS-HT DM mice.
Oxidative stress was potentiated in diabetic BIS-HT mice. The time course of 24 h urinary isoprostane over 20 weeks in control mice (circles) and after the induction of diabetes (squares) in BIS-WT (white) and BIS-HT (black) mice (a). The effect of tempol on the urinary isoprostane level at 20 weeks after diabetes induction (b). Serum and 24 h urinary 8-OH-dG levels (c, d) were determined at 20 weeks of the study. n = 6–8/group. The values represent the mean ± SE of 6–8 mice/group. ***p < 0.001 vs corresponding BIS-WT mice. ††† p < 0.001 vs BIS-HT DM mice without tempol treatment. DM, diabetes; temp, tempol
Comparison of ROS levels in BIS-WT (white) and BIS-HT (black) mice. Representative DHE staining for superoxide production in control and diabetic BIS-WT and BIS-HT mice (a; ×200). Fluorescence intensity was about twofold higher in BIS-HT DM mice compared with BIS-WT DM mice in the quantitative determination (c). Representative photomicrographs of glomerular nitrotyrosine expression (b; original magnification, × 1000) and quantitative analysis (d) indicated that peroxynitrite level was about threefold higher in BIS-HT DM mice than BIS-WT DM mice. n = 3–6/group. ***p < 0.001 vs BIS-WT DM mice. DM, diabetes
To verify whether the acceleration of diabetic nephropathy in BIS-HT mice could be attributed to increased oxidative stress, we examined the effects of antioxidant on renal function and oxidative damage in diabetic BIS-WT and BIS-HT mice. Based on the increases in isoprostane and albuminuria, we investigated the effect of tempol, a SOD mimetic, which was administered 12 weeks after the induction of diabetes. As shown in Fig. 2, tempol treatment over the course of 8 weeks resulted in significant downregulation of serum 8-OH-dG concentrations as well as urinary 8-OH-dG and 8-isoprostane concentrations to the corresponding levels in control BIS-HT mice. These changes in oxidative markers correlated with a decrease in albuminuria in diabetic BIS-HT mice administered with tempol, from 78 to 22 μg/24 h (Fig. 1d). In addition, tempol treatment at 12 weeks after the induction of diabetes restored the kidney-to-body-weight ratios, serum creatinine levels and creatinine clearance to the corresponding levels in control BIS-HT mice, though the level of hyperglycaemia remained unchanged (Table 1 and Fig. 1a, b). These findings suggest that aggravated renal damage in BIS-HT DM mice is associated with an increased susceptibility to oxidative stress, which can be ameliorated by antioxidant treatment.
Effects of BIS haplotype and tempol on renal histopathology
Histological analyses using light microscopy showed that there were no apparent differences between control BIS-WT and BIS-WT DM mice with respect to mesangial fractional areas and tubulointerstitial fibrosis (Fig. 4). Quantitative analyses also indicated that the mesangial fractional areas and degree of tubulointerstitial fibrosis were not significantly higher in BIS-WT DM mice than in BIS-WT mice. In contrast, the mesangial fractional areas and tubulointerstitial fibrosis in BIS-HT DM mice were 2.5 and 3.7 times greater, respectively, than those in BIS-HT mice; these differences were statistically significant (p < 0.001 for both). Consistent with the change in the mesangial fractional area, the expression of pro-fibrotic growth factors TGF-β1 and HIF-1α [29], together with levels of extracellular matrix type IV collagen (Col IV) and F4/80-positive inflammatory cells in the glomerulus and tubulointerstitial area were significantly higher in BIS-HT DM mice compared with BIS-HT and BIS-WT DM mice (Fig. 5). All the excess phenotypes of diabetes-induced renal changes and inflammation seen in BIS-HT DM mice, even under experimentally established diabetic nephropathy, were recovered by the tempol treatment (Figs 4, 5).
Renal histopathology of the glomerulus and tubulointerstitial areas. Representative image of PAS (a; original magnification, ×400) and Masson’s trichrome stain (c; original magnification, ×100) from renal tissue as indicated. Quantitative assessment of the extracellular matrix area in the glomerulus (b) and fibrosis in the tubulointerstitial space (d) from indicated groups, n = 6–8/group; white, BIS-WT; black, BIS-HT. *p < 0.05 and ***p < 0.001 vs corresponding BIS-WT mice. ††† p < 0.001 vs BIS-HT DM mice without tempol. DM, diabetes; temp, tempol
Immunohistochemistry for pro-fibrotic and pro-inflammatory factors. Immunohistochemical staining was performed for TGF-β1 (b), Col IV (c), HIF-1α (d) and F4/80 (e) in the glomerulus of control and diabetic BIS-WT (white) and BIS-HT (black) mice with or without tempol treatment (a; original magnification, ×400). Quantitative assessment of TGF-β1, Col IV, HIF-1α and F4/80 immunostaining in the glomeruli of the indicated groups (b–e). Data are shown as fold change compared with mean intensities of control BIS-WT mice. For F4/80, the positive cells per glomerulus were presented, n = 6–8/group; ***p < 0.001 vs BIS-WT DM mice. ††† p < 0.001 vs BIS-HT DM mice without tempol treatment. DM, diabetes; temp, tempol
We also investigated the degree of apoptosis in the kidneys from the four experimental groups. Compared with other groups, BIS-HT DM mice showed a significant increase in the number of TUNEL- and active caspase-3-positive cells in the glomerulus and cortical tubular areas (ESM Fig. 1). Tempol treatment also reduced the proportion of apoptotic cells in BIS-HT DM mice compared with BIS-HT mice. Therefore, decreased BIS expression also resulted in increased apoptosis in diabetic kidneys, which is associated with an increase in oxidative stress.
Impaired induction of SOD in BIS-HT DM mice
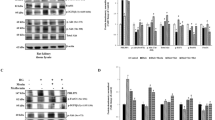
Intracellular ROS levels are maintained by the balance between ROS production and antioxidant capacity. Figure 6a shows that the production of NOX-4, which has been implicated as the main superoxide-producing enzyme in the kidney [30, 31], was not notably increased by diabetes induction in either BIS-WT or BIS-HT mice. NOX-1 levels were increased in diabetic BIS-WT and BIS-HT mice to a similar extent (1.6- and 1.4-fold increases vs controls, respectively). The densitometric analysis also indicated that there were no significant differences in the expression of p22phox and RAC1 proteins, the catalytic components of the NOX family, between control and diabetes groups for both BIS-WT and BIS-HT mice. We then examined whether BIS deficiency affects the expression or activity of SOD, the primary antioxidant defence enzyme, on high-glucose-induced oxidative stress in the kidneys of BIS-HT mice. Figure 6b shows that production of SOD1 was 2.4-fold higher in BIS-WT DM mice compared with control BIS-WT mice (p < 0.05). SOD2 production was slightly higher in BIS-WT DM mice (about 1.3-fold) compared with BIS-WT mice. However, in BIS-HT mice, the levels of both SOD1 and SOD2 were not increased, but rather decreased, by the induction of diabetes (0.9- and 0.8-fold compared with controls, respectively). Notably, the basal expression levels of SOD1 and SOD2 in control BIS-HT mice were higher than those in the control BIS-WT mice (2.8- and 1.5-fold, respectively). In addition, the total SOD activity was 2.3-fold higher in BIS-WT DM mice than in control BIS-WT mice (284 vs 125 U/mg), whereas SOD activity was not substantially different between BIS-HT DM and control BIS-HT mice (141 vs 161 U/mg), which is also consistent with the western blot result (Fig. 6c). Thus, these results indicate that impaired induction of SOD expression, rather than increased induction of NOX, in response to hyperglycaemic oxidative stress, is responsible for the increased susceptibility to oxidative stress in BIS-HT DM mice.
Levels of NOX and SOD proteins and determination of total SOD activity in diabetic kidney from BIS-WT and BIS-HT mice. Western blot analysis of NOX-1, NOX-4, p22phox and RAC1 proteins (a) and SOD1 and SOD2 (b) in renal tissues from control or diabetic BIS-WT and BIS-HT at 20 weeks after the induction of diabetes. The results from two mice per indicated group are shown. The total SOD activity was measured in kidney from control and diabetic BIS-WT and BIS-HT mice using a commercial kit and the mean values are presented with ± SE (c; n = 5, BIS-WT, white; n = 4, BIS-HT mice, black). ***p < 0.001 vs BIS-WT DM mice. ††† p < 0.001 vs control BIS-WT mice. DM, diabetes
BIS modulates expression of SOD1 and SOD2 induced by high glucose in NMS mesangial cells and HK-2 kidney tubular cells
To verify whether the impaired induction of SOD activity on induction of diabetes in the kidneys of BIS-HT mice is the direct result of a BIS deficiency, we performed in vitro experiments using an siRNA strategy and high-glucose treatment. High-glucose treatment resulted in an increase in SOD activity in both control NMS and HK-2 cells in a time-dependent manner up to 72 h, as observed in BIS-WT and BIS-WT DM mice. However, downregulation of BIS resulted in impaired induction of SOD activity and notable decreases in SOD activity, by 42% and 41%, respectively, after 72 h of high-glucose treatment in NMS and HK-2 cells, compared with activity in control cells (Fig. 7a, e). It is noteworthy that the basal SOD activity was higher in BIS-knockdown NMS and HK-2 cells than in control NMS and HK-2 cells, as BIS-HT mice had higher basal SOD activity than BIS-WT mice (Fig. 6c). Western blot assays and densitometric analyses showed that production of SOD1 and SOD2 protein in both control NMS and HK-2 cells was induced by exposure to high glucose concentrations in a time-dependent manner, and correlated with an increase in SOD activity (Fig. 7b–d, f–h). NMS and HK-2 cells in which BIS expression was suppressed showed similar SOD2 expression patterns but a retarded profile, compared with control cells. The increasing pattern of SOD1 expression was not observed in BIS-knockdown NMS and HK-2 cells. The same concentration of mannitol had no significant effect on the induction of SOD1 or SOD2 in either cell line, and was not suppressed by BIS knockdown (ESM Fig. 2). Densitometric analysis revealed that the SOD1 expression profile in BIS-knockdown NMS and HK-2 cells showed a pattern of slightly decreased levels after high-glucose treatment, similar to the profile of SOD activity. The suppression of BIS expression had no effect on the transcript levels of Sod1 and Sod2 in NMS and HK-2 cells (ESM Fig. 3). Thus, these results suggest that BIS deficiency modulates the protein stability of SOD1 and SOD2 on glucose-induced oxidative stress rather than the transcription rates or mRNA stability of Sod1 and Sod2. Finally, we demonstrated that the ROS accumulation caused by high-glucose treatment was greatly enhanced by BIS knockdown in NMS and HK-2 cells (p < 0.001 for both cells, Fig. 8), as observed in the renal tissues of BIS-HT DM mice (Fig. 3). Control NMS and HK-2 cells also showed an increase in ROS levels of 4.5 and 2.6-fold, respectively, on high-glucose treatment, considerably less than for Bis-siRNA-treated cells.
Effect of BIS knockdown on SOD activity and expression in mesangial and tubular cells. NMS mesangial (a–d) and HK-2 tubular cells (e–h) were transfected with control (white) or Bis-specific siRNA (black) and subsequently treated with additional glucose to final concentrations of 25 mmol/l and 50 mmol/l glucose, respectively, for 72 h. Total SOD activity was determined at each time point and compared with that of normal glucose (a, e; n = 4). Representative image of western blot assay for SOD1 and SOD2 in NMS mesangial cells and HK-2 tubular cells after BIS knockdown followed by high glucose treatment (b, f). Densitometric analysis for SOD1 and SOD2 was presented as fold change compared with the value with normal glucose (c, d, g, h; n = 5 for NMS cells, n = 4 for HK-2 cells). *p < 0.05 vs the value for normal glucose in each group. HG, high glucose; NG, normal glucose
ROS levels in mesangial and tubular cells exposed to high glucose. BIS production was suppressed in NMS and HK-2 cells and exposed to high glucose for 48 h as in Fig. 7. Cellular ROS levels with normal glucose (white) and high glucose (grey) were assessed by DCF-DA staining and subsequent FACS analysis. The representative histograms and mean fluorescence intensities from triplicate experiments in each group are shown. *p < 0.05 and ***p < 0.001 vs the value with normal glucose in each group. HG, high glucose; NG, normal glucose
Discussion
From the significant increase in albuminuria and decrease in the glomerular filtration rate, which are two major clinical features of diabetic nephropathy, BIS-HT mice were found to exhibit more severe STZ-induced diabetic nephropathy than BIS-WT mice (Fig. 1). Furthermore, compared with the findings for the BIS-WT DM mice, the significant increase in oxidative DNA damage and lipid peroxidation as well as enhanced ROS accumulation in BIS-HT DM mice indicate that BIS-HT mice were exposed to increased oxidative stress induced by hyperglycaemia (Figs 2, 3). The oxidative damage in BIS-HT DM mice was closely related to the glomerulosclerosis associated with increases in TGF-β1, Col IV and HIF-1 α expression in the glomerulus as well as tubulointerstitial fibrosis (Figs 4, 5). Our results suggest that the increased oxidative stress in BIS-HT DM mice may be the molecular basis for severe diabetic nephropathy. This presumption was confirmed by the restoration of all the severe renal phenotypes of BIS-HT DM mice by treatment of tempol. The experiments with tempol demonstrated that, even in the case of advanced diabetic nephropathy, antioxidant treatment could slow the progress of renal damage and subsequently restore renal phenotypes. We also demonstrated that hyperglycaemia in BIS-haploinsufficient mice caused apoptosis in subpopulations of mesangial and tubular cells with increased caspase-3 activity (ESM Fig. 1). Apoptosis in BIS-HT DM mice was efficiently suppressed by the tempol treatment, indicating that the accumulation of ROS is the primary event leading to apoptosis.
Increased oxidative stress in diabetic nephropathy could be attributed to an imbalance between ROS production and its removal capacity. Although multiple enzymatic pathways have been implicated in the generation of ROS in hyperglycaemia, NOX might be an important ROS source in diabetic kidney [32]. In particular, NOX-4 levels were shown to increase early in the STZ-induced diabetic rat model and NOX inhibition prevented diabetic changes of kidney [30, 31, 33]. On the other hand, the central role of SOD in diabetic nephropathy has been also demonstrated in previous studies. Notably, in mice, the overexpression of SOD has been found to abrogate diabetes-induced renal injuries, most likely due to reduced superoxide–NO interactions [9]. In addition, ablation of the Sod1 gene has been found to result in an acceleration of diabetic renal injuries in both STZ-induced C57BL/6 strains and in C57BL/6-Akita strains [34, 35]. Recently, a human genetic study revealed that diabetic risk is correlated with polymorphisms in SOD1 and SOD2 genes [36–38]. In the present study, we demonstrate that total SOD activity and SOD expression were enhanced in BIS-WT DM mice, but not BIS-HT DM mice, compared with normal WT mice, while NOX expression as well as catalytic protein levels were not notably different between BIS-WT and BIS-HT mice with or without diabetes (Fig. 6). Moreover, suppression of BIS expression resulted in an impaired induction of SOD activity as well as SOD expression on treatment with high glucose concentrations in proximal tubule or mesangial cells in vitro (Fig. 7). These findings corroborate the results of our previous study, which showed that the downregulation of BIS sensitised glioma cells to hypoxic stress, accompanied by an impaired induction of SOD activity [23]. Therefore, we speculate that BIS is required for the induction or maintenance of SOD expression against oxidative stress, rather than the modulation of NOX expression, under pathophysiological conditions. Accordingly, BIS deficiency may result in decreased SOD expression and SOD activity, limiting the removal of excess ROS induced by chronic hyperglycaemia. Our results support the critical role of renal SOD activity as a primary antioxidant defence for high-glucose-induced ROS, which eventually determines the progress of overt diabetic nephropathy. However, a more comprehensive study on NOX and related proteins using renal tissues from earlier time points after diabetes induction should be performed to clarify the possible role for BIS in the mitochondria-derived ROS production at early stages in the progression of diabetes.
Although our results demonstrate that the induction of SOD expression and activity is influenced by a BIS deficiency in BIS-HT mice as well as the in vitro response of NMS and HK-2 cells to glucose-induced oxidative stress, the mechanism by which BIS regulates SOD induction remains unclear. In BIS-expressing glioma cells, the induction of SOD1 mRNA has previously been found to be impaired on hypoxic stress, which correlates with SOD activity [23]. However, in the present study, the transcript levels of Sod1 and Sod2 were not affected by BIS knockdown in HK-2 cells as well as in NMS cells, and SOD1 and SOD2 protein expression was impaired or delayed by glucose treatment compared with the patterns of increasing expression observed in control cells. The induction of total SOD activity in NMS and HK-2 cells was abrogated by BIS knockdown, correlating with decreased SOD1 expression compared with that of SOD2. Taken together, BIS primarily regulates SOD1 induction in response to high-glucose-induced oxidative stress by modulating the translation or degradation of SOD1 protein rather than its transcriptional levels, which requires a different mechanism as indicated by previous observations of glial cells subjected to hypoxic stress.
In the case of pro-oxidant or aggregation-prone status, BIS has been suggested to interact with HSP70 to activate macroautophagy, whereas interaction of BAG1 with HSP70 is known to be essential for effective proteasomal degradation [16, 22]. The misfolding and aggregation of SOD have been shown to increase with oxidative stress in neuronal cells [39]. Thus, it can be simply speculated that, with deficiency of BIS, BAG1 may replace BIS in the formation of complex with HSP70 and facilitate the proteasome-dependent degradation of SOD, resulting in impaired induction of SOD1 or SOD2 expression. Supporting this idea, BIS has been recently reported to stabilise myeloid cell leukaemia sequence 1 (BCL2-related) (MCL-1), preventing its HSP70-dependent degradation [40]. However, the constitutive expression of SOD1, as well as SOD2, was higher in BIS-HT mice than in BIS-WT mice before induction of diabetes, which correlates with basal SOD activity. BIS knockdown in NMS and HK-2 cells also resulted in increased basal SOD activity. Thus, the constitutive expression and activity of SOD appear to be modulated through a different mechanism that involves inducible expression in response to oxidative stress.
In conclusion, our results indicate that diabetic nephropathy is aggravated in the condition of BIS haploinsufficiency, probably through inadequate induction or maintenance of SOD expression, suggesting that the maintenance or enhancement of BIS expression in the kidney is a potential therapeutic option for treating diabetes-induced oxidative renal damage. In addition, the BIS-HT mice, which exhibit normal phenotypic growth and reproduction in the physiological conditions, could be used as an aggressive diabetic mouse model for the development of an intervention strategy to slow the progress of diabetic nephropathy.
Abbreviations
- BAG:
-
BCL2-associated athanogene
- BCL-2:
-
B cell CLL/lymphoma 2
- BIS:
-
BCL-2 interacting cell death suppressor
- BIS-HT(-DM):
-
Bis-heterozygote (with diabetes)
- BIS-WT(-DM):
-
Bis-wild-type (with diabetes)
- Col IV:
-
Type IV collagen
- DCF-DA:
-
2′7′-Dichlorofluorescein diacetate
- DHE:
-
Dihydroethidine
- F4/80:
-
Cell surface glycoprotein F4/80
- HIF-1α:
-
Hypoxia-inducible factor 1-α
- HK-2:
-
Human kidney-2 [cells]
- HSP70:
-
Heat shock 70 kDa protein
- NOX:
-
NADPH oxidase
- NMS:
-
Normal mesangial [cells]
- 8-OH-dG:
-
8-Hydroxy-deoxyguanosine
- RAC1:
-
Ras-related C3 botulinum toxin substrate 1
- ROS:
-
Reactive oxygen species
- si:
-
Small interfering
- SOD:
-
Superoxide dismutase
- STZ:
-
Streptozotocin
- Tempol:
-
4-Hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl
References
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625
Brownlee M (2007) Preventing kidney cell suicide. Nat Med 13:1284–1285
Wagener FA, Dekker D, Berden JH, Scharstuhl A, van der Vlag J (2009) The role of reactive oxygen species in apoptosis of the diabetic kidney. Apoptosis 14:1451–1458
Higgins DF, Kimura K, Bernhardt WM et al (2007) Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117:3810–3820
Neusser MA, Lindenmeyer MT, Moll AG et al (2010) Human nephrosclerosis triggers a hypoxia-related glomerulopathy. Am J Pathol 176:594–607
Brezniceanu ML, Liu F, Wei CC et al (2008) Attenuation of interstitial fibrosis and tubular apoptosis in db/db transgenic mice overexpressing catalase in renal proximal tubular cells. Diabetes 57:451–459
Brezniceanu ML, Liu F, Wei CC et al (2007) Catalase overexpression attenuates angiotensinogen expression and apoptosis in diabetic mice. Kidney Int 71:912–923
Craven PA, Melhem MF, Phillips SL, DeRubertis FR (2001) Overexpression of Cu2+/Zn2+ superoxide dismutase protects against early diabetic glomerular injury in transgenic mice. Diabetes 50:2114–2125
DeRubertis FR, Craven PA, Melhem MF, Salah EM (2004) Attenuation of renal injury in db/db mice overexpressing superoxide dismutase: evidence for reduced superoxide-nitric oxide interaction. Diabetes 53:762–768
Lee JH, Takahashi T, Yasuhara N, Inazawa J, Kamada S, Tsujimoto Y (1999) BIS, a Bcl-2-binding protein that synergizes with Bcl-2 in preventing cell death. Oncogene 18:6183–6190
Bonelli P, Petrella A, Rosati A et al (2004) BAG3 protein regulates stress-induced apoptosis in normal and neoplastic leukocytes. Leukemia 18:358–360
Liu P, Xu B, Li J, Lu H (2009) BAG3 gene silencing sensitizes leukemic cells to bortezomib-induced apoptosis. FEBS Lett 583:401–406
Romano MF, Festa M, Petrella A et al (2003) BAG3 protein regulates cell survival in childhood acute lymphoblastic leukemia cells. Cancer Biol Ther 2:508–510
Rosati A, Ammirante M, Gentilella A et al (2007) Apoptosis inhibition in cancer cells: a novel molecular pathway that involves BAG3 protein. Int J Biochem Cell Biol 39:1337–1342
Carra S, Brunsting JF, Lambert H, Landry J, Kampinga HH (2009) HspB8 participates in protein quality control by a non-chaperone-like mechanism that requires eIF2α phosphorylation. J Biol Chem 284:5523–5532
Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C (2009) Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J 28:889–901
Chen L, Wu W, Dentchev T et al (2004) Light damage induced changes in mouse retinal gene expression. Exp Eye Res 79:239–247
Crippa V, Sau D, Rusmini P et al (2010) The small heat shock protein B8 (HspB8) promotes autophagic removal of misfolded proteins involved in amyotrophic lateral sclerosis (ALS). Hum Mol Genet 19:3440–3456
Lee MY, Kim SY, Shin SL et al (2002) Reactive astrocytes express bis, a bcl-2-binding protein, after transient forebrain ischemia. Exp Neurol 175:338–346
Pagliuca MG, Lerose R, Cigliano S, Leone A (2003) Regulation by heavy metals and temperature of the human BAG-3 gene, a modulator of Hsp70 activity. FEBS Lett 541:11–15
Jacobs AT, Marnett LJ (2009) HSF1-mediated BAG3 expression attenuates apoptosis in 4-hydroxynonenal-treated colon cancer cells via stabilization of anti-apoptotic Bcl-2 proteins. J Biol Chem 284:9176–9183
Doong H, Rizzo K, Fang S, Kulpa V, Weissman AM, Kohn EC (2003) CAIR-1/BAG-3 abrogates heat shock protein-70 chaperone complex-mediated protein degradation: accumulation of poly-ubiquitinated Hsp90 client proteins. J Biol Chem 278:28490–28500
Jung SE, Kim YK, Youn DY et al (2010) Down-modulation of BIS sensitizes cell death in C6 glioma cells induced by oxygen-glucose deprivation. Brain Res 1349:1–10
Youn DY, Lee DH, Lim MH et al (2008) BIS deficiency results in early lethality with metabolic deterioration and involution of spleen and thymus. Am J Physiol Endocrinol Metab 295:E1349–E1357
Youn DY, Yoon JS, Kim YK et al (2011) Deletion of the bis gene results in a marked increase in the production of corticosterone that is associated with thymic atrophy in mice. Am J Physiol Endocrinol Metab 301:E223–E231
Kim MY, Lim JH, Youn HH et al (2013) Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1alpha axis in db/db mice. Diabetologia 56:204–217
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Trujillo M, Ferrer-Sueta G, Radi R (2008) Peroxynitrite detoxification and its biologic implications. Antioxid Redox Signal 10:1607–1620
Haase VH (2009) Pathophysiological consequences of HIF activation: HIF as a modulator of fibrosis. Ann NY Acad Sci 1177:57–65
Etoh T, Inoguchi T, Kakimoto M et al (2003) Increased expression of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia 46:1428–1437
Gorin Y, Block K, Hernandez J et al (2005) Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280:39616–39626
Gill PS, Wilcox CS (2006) NADPH oxidases in the kidney. Antioxid Redox Signal 8:1597–1607
Sedeek M, Gutsol A, Montezano AC et al (2013) Renoprotective effects of a novel Nox1/4 inhibitor in a mouse model of type 2 diabetes. Clin Sci (Lond) 124:191–202
DeRubertis FR, Craven PA, Melhem MF (2007) Acceleration of diabetic renal injury in the superoxide dismutase knockout mouse: effects of tempol. Metabolism 56:1256–1264
Fujita H, Fujishima H, Takahashi K et al (2012) SOD1, but not SOD3, deficiency accelerates diabetic renal injury in C57BL/6-Ins2(Akita) diabetic mice. Metabolism 61:1714–1724
Mollsten A, Marklund SL, Wessman M et al (2007) A functional polymorphism in the manganese superoxide dismutase gene and diabetic nephropathy. Diabetes 56:265–269
Lee SJ, Choi MG, Kim DS, Kim TW (2006) Manganese superoxide dismutase gene polymorphism (V16A) is associated with stages of albuminuria in Korean type 2 diabetic patients. Metabolism 55:1–7
Al-Kateb H, Boright AP, Mirea L et al (2008) Multiple superoxide dismutase 1/splicing factor serine alanine 15 variants are associated with the development and progression of diabetic nephropathy: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Genetics study. Diabetes 57:218–228
Ezzi SA, Urushitani M, Julien JP (2007) Wild-type superoxide dismutase acquires binding and toxic properties of ALS-linked mutant forms through oxidation. J Neurochem 102:170–178
Boiani M, Daniel C, Liu X, Hogarty MD, Marnett LJ (2013) The stress protein BAG3 stabilizes Mcl-1 protein and promotes survival of cancer cells and resistance to antagonist ABT-737. J Biol Chem 288:6980–6990
Funding
This research was supported by Basic Science Research Programs through the National Research Foundation of Korea (NRF), funded by the Minister of Education, Science and Technology (2012R1A1A2007589 and 2012R1A5A2047939) and a grant from the Korean Health Technology R&D Project, Minister of Health and Welfare, Republic of Korea (A111055).
Contribution statement
CWP and J-HL developed the concept and design of the research, analysed and interpreted the data and edited the manuscript. JHL, D-YY, HJY, HHY and MYK contributed to acquisition of data and drafting the manuscript. SC, Y-SK and YSC contributed to analysis and interpretation of data and revising the manuscript. All authors approved the final version to be published.
Duality of interest
All authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ji Hee Lim and Dong-Ye Youn contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
(PDF 221 kb)
ESM Fig. 2
(PDF 118 kb)
ESM Fig. 3
(PDF 97 kb)
ESM Table 1
(PDF 93 kb)
ESM Methods
(PDF 105 kb)
Rights and permissions
About this article
Cite this article
Lim, J.H., Youn, DY., Yoo, H.J. et al. Aggravation of diabetic nephropathy in BCL-2 interacting cell death suppressor (BIS)-haploinsufficient mice together with impaired induction of superoxide dismutase (SOD) activity. Diabetologia 57, 214–223 (2014). https://doi.org/10.1007/s00125-013-3064-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-3064-0