Abstract
Aims/hypothesis
Overproduction of phosphoprotein enriched in diabetes (PED, also known as phosphoprotein enriched in astrocytes-15 [PEA-15]) is a common feature of type 2 diabetes and impairs insulin action in cultured cells and in mice. Nevertheless, the potential role of PED in diabetic complications is still unknown.
Methods
We studied the effect of PED overproduction and depletion on kidney function in animal and cellular models.
Results
Transgenic mice overexpressing PED (PEDTg) featured age-dependent increases of plasma creatinine levels and urinary volume, accompanied by expansion of the mesangial area, compared with wild-type littermates. Serum and kidney levels of TGF-β1 were also higher in 6- and 9-month-old PEDTg. Overexpression of PED in human kidney 2 cells significantly increased TGF-β1 levels, SMAD family members (SMAD)2/3 phosphorylation and fibronectin production. Opposite results were obtained following genetic silencing of PED in human kidney 2 cells by antisense oligonucleotides. Inhibition of phospholipase D and protein kinase C-β by 2-butanol and LY373196 respectively reduced TGF-β1, SMAD2/3 phosphorylation and fibronectin production. Moreover, inhibition of TGF-β1 receptor activity and SMAD2/3 production by SB431542 and antisense oligonucleotides respectively reduced fibronectin secretion by about 50%. TGF-β1 circulating levels were significantly reduced in Ped knockout mice and positively correlated with PED content in peripheral blood leucocytes of type 2 diabetic patients.
Conclusions/interpretation
These data indicate that PED regulates fibronectin production via phospholipase D/protein kinase C-β and TGF-β1/SMAD pathways in kidney cells. Raised PED levels may therefore contribute to the abnormal accumulation of extracellular matrix and renal dysfunction in diabetes.
Similar content being viewed by others
Introduction
Diabetic nephropathy is a frequent complication of type 1 and type 2 diabetes mellitus and is currently considered the leading cause of end-stage renal disease [1]. While thickening of the glomerular basement membrane, glomerular hypertrophy and mesangial expansion are well known features of diabetic nephropathy, the pathogenesis of these alterations is not very clear yet. The DCCT, UK Prospective Diabetes Study (UKPDS) and Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) studies have shown the role of hyperglycaemia as a causative factor in the development and the progression of diabetic nephropathy [2–5]. Nevertheless, hyperglycaemia alone is clearly not sufficient to account for the heterogeneity and variability of the clinical appearance of the disorder. Indeed, accumulating evidence points to critical genetic factors predisposing only a subset of patients with diabetes to nephropathy [6–8].
Several studies have shown an important involvement of growth factors and cytokines [9, 10]. In particular, the TGF-β1 is a key factor in experimental models of diabetic kidney disease as well as in patients with diabetic nephropathy [11–15]. In fact, TGF-β1 levels are increased in diabetic patients and high glucose levels upregulate expression and bioactivity of TGF-β1 in almost all renal cell types [11, 12, 16, 17]. Upon binding to its receptor, TGF-β1 phosphorylates the receptor-regulated SMAD family members (SMAD)2 and 3, which form oligomeric complexes with the common SMAD (SMAD 4). These complexes then translocate into the nucleus, thereby regulating transcription of target genes, including those encoding type I and type IV collagen, laminin and fibronectin [14, 16, 18, 19].
Phosphoprotein enriched in diabetes (PED, also known as phosphoprotein enriched in astrocytes-15 [PEA-15]) is a scaffold cytosolic protein originally identified as a major astrocyte phosphoprotein and found to be widely present in different tissues [20, 21]. PED plays an important role in mitogenic and metabolic signalling [21–23]. Gene profiling studies have shown that PED (also known as PEA15) is commonly overexpressed in individuals with type 2 diabetes [21, 24]. In cultured muscle and adipose cells and in peripheral tissues from transgenic mice, forced production of PED impairs insulin-stimulated GLUT4 translocation and glucose transport, suggesting that PED may contribute to insulin resistance in type 2 diabetes [25]. Further studies have shown that PED stabilises phospholipase D (PLD) [26] and induces activation of classical protein kinase C (PKC) isoforms, including PKCα and PKCβ [23, 27]. The induction of classical PKCs, in turn, inhibits insulin signalling, at least in skeletal muscle, adipose and liver cells [28–31]. Nevertheless, the role of PED in diabetic complications is not known.
In the present work we addressed the question of whether PED overproduction determines abnormalities in kidney function and whether it may represent an initial defect in the progression toward diabetic nephropathy.
Methods
Materials
Media, sera, antibiotics for cell culture and the lipofectamine reagent were from Invitrogen (Grand Island, NY, USA). The anti-PED polyclonal rabbit antibody and pcDNA3PED vector have been previously described [21]. pSMAD2/3 (Ser433/435), SMAD2, PKCα, PKCβ, fibronectin, laminin and collagen I and IV antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). SMAD3 was purchased from Calbiochem (San Diego, CA, USA). Phosphorothioate oligonucleotides antisense sequences used were as follows: SMAD2 AS: 5′-GCACGATGGACGACAT-3′; SMAD2 S: 5′-CAATGCGAGTACGCGA-3′; SMAD3 AS: 5′-GCAGGATGGACGACAT-3′; SMAD3 S: 5′-GGAGTCAGACTGACGA-3′; PED antisense oligonucleotides were as previously described [25]. Protein electrophoresis reagents were purchased from Bio-Rad (Richmond, VA, USA), and western blotting and enhanced chemiluminescence (ECL) reagents from Amersham Biosciences (Arlington Heights, IL, USA). All other chemicals were from Sigma Aldrich (St Louis, MO, USA).
Mouse phenotyping
Generation of transgenic mice overexpressing PED (PEDTg) [25] and of Ped/Pea-15 knockout mice (Ped-KO) bearing ubiquitous ablation of the gene [32] has been previously described. Homozygous Ped-KO were used for the study. Both PEDTg and Ped-KO mice were fertile. Body weight of PEDTg was comparable to that of wild-type littermates, while, as previously described [32], Ped-KO displayed a slightly lower body weight. All procedures described below were approved by the Institutional Animal Care and Utilisation Committee. Animals were kept in a 12-h dark–light cycle and had free access to standard diet. Mice chosen for experimentation were randomly selected from each box of mice housed in groups of three to four. Mice were habituated in individual metabolism cages (Lenderking Caging Products, Millersville, MD, USA) for 24 h. Then, the following variables were analysed: food intake (g/24 h), water intake (ml/24 h), urine volume excretion (ml/24 h), urine specific gravity, urine pH, urine glucose (mg/24 h) and albumin excretion (μg/24 h). The measurements of blood pressure and heart rate were performed partially modifying previously described methods [33]. Briefly, a 1.0-Fr polyimide pressure catheter (SPR 1000/2; Millar Instruments, Houston, TX, USA) was inserted into the left carotid artery and advanced into the ascending aorta of anaesthetised mice (2% isoflurane, 98% oxygen). Blood pressure and heart rate were recorded for 15 min after suspending isoflurane administration with an 8 channel recorder (Gould Instruments Systems, Cleveland, OH, USA). Data were analysed using Powerlab and Chart 5 software (AD Instruments, Sydney, NSW, Australia).
Renal histology and morphometric analysis
Kidney sections were fixed by immersion in Carnoy solution followed by 4% buffered formaldehyde (vol./vol.) and embedded in paraffin. The fixed, embedded kidneys were cut into 2 μm sections and stained with periodic acid–Schiff’s reagent. To quantify mesangial expansion, sections were coded and examined by light microscopy by two observers unaware of the experimental protocol applied. According to previous reports [34, 35], measurement of the mesangial area of 30 glomeruli randomly selected in each mouse by scanning of the outer cortex was performed with a computer-aided manipulator (KS-400; Carl Zeiss Vision, Munich, Germany)
Measurement of serum TGF-β1 and urine albumin
Blood samples of mice were collected from the orbital sinus under anaesthesia. After centrifugation (800×g) of the blood samples, TGF-β1 levels in the supernatant fractions were measured using ELISA kits (R&D System, Minneapolis, MN, USA). The same kits were used to measure TGF-β1 levels in the media of the cells. Samples of urine collected through metabolism cages (five samples for each animal; ten animals/group) were briefly centrifuged at 500×g and then albumin concentration was determined using ELISA kits (Bethyl Laboratories, Montgomery, TX, USA).
Tissue collection and primary mouse tubular kidney cell cultures
Tissue samples (kidney) were collected rapidly after mice were killed by pentobarbitone overdose. Tissues were snap-frozen in liquid nitrogen and stored at −80°C for subsequent western blot analysis. Mouse tubular epithelial cells were isolated as previously described [36]. Cells were grown until confluent (8 to 12 days) in RPMI 1640 medium supplemented with 20% fetal calf serum, 2% (wt/vol.) l-glutamine, 20,000 units/ml penicillin, 20,000 μg/ml streptomycin at 37°C in 5% CO2.
Cell culture procedures and transfection
Human kidney 2 (HK2) proximal tubular cells were cultured in RPMI 1640 medium (Invitrogen), containing 11.2 mmol/l glucose supplemented with 10% fetal calf serum, 2% (wt/vol.) l-glutamine, 10,000 units/ml penicillin, 10,000 μg/ml streptomycin at 37°C in 5% (vol./vol.) CO2. Stable transfection of PED cDNA and transient transfection of antisense oligonucleotides [37] were performed by the lipofectamine method according to the manufacturer’s instructions (Invitrogen). For these studies, 60 to 80% confluent cells were washed twice with Optimem (Invitrogen) and incubated for 8 h with 5 μg of plasmid construct or antisense oligonucleotides and 45 to 60 μl of lipofectamine reagent. The medium was then replaced with DMEM with 10% (vol./vol.) fetal calf serum and cells further incubated for 15 h before being assayed. Transfection efficiency for antisense oligonucleotides was estimated as 45 ± 10% by co-transfection with green fluorescent protein.
Tissue and cell lysates and immunoblotting
Tissue samples were homogenised in a Polytron (Brinkman Instruments, Westbury, NY, USA) in 20 ml T-PER reagent (Pierce, Rockford, IL, USA) per gram of tissue according to manufacturer’s instructions. After centrifugation at 5000×g for 5 min, supernatant fraction was collected. Cells were solubilised in lysis buffer (50 mmol/l HEPES, pH 7.5, 150 mmol/l NaCl, 10 mmol/l EDTA, 10 mmol/l Na4P2O7, 2 mmol/l Na3VO4, 100 mmol/l NaF, 10% (vol./vol.) glycerol, 1% (vol./vol.) Triton X-100, 1 mmol/l PMSF, 10 mg/ml aprotinin) for 1 h at 4°C and lysates were centrifuged at 5,000×g for 20 min. Total homogenates were separated by SDS-PAGE and transferred on to 0.45 μm Immobilon-P membranes as previously described [38]. Upon incubation with primary and secondary antibodies, immunoreactive bands were detected by ECL according to the manufacturer's instructions.
For peripheral blood leucocyte (PBL) separation, EDTA-treated whole-blood samples were first centrifuged for 10 min at 300×g and the plasma removed. PBLs were separated using a 6% (vol./vol.) dextran gradient in filtered PBS, pH 7.4, as previously described [24], washed three times in PBS, counted and resuspended in 1 ml of PBS for subsequent use.
Real-time RT-PCR analysis
Total cellular RNA was isolated from whole kidneys of wild-type and PEDTg mice using a kit (RNeasy; Qiagen, Hilden, Germany) according to manufacturer’s instructions. Tissue or cell RNA (1 μg) was reverse-transcribed using Superscript II Reverse Transcriptase (Invitrogen). PCR reactions were analysed using SYBR Green mix (Invitrogen). Reactions were performed using Platinum SYBR Green qPCR Super-UDG and a multicolour real-time PCR detection system (Cycler IQ; Bio-Rad). All reactions were performed in triplicate and β-actin was used as an internal standard. Primer sequences used were as follows: TGF-β1 (forward) 5′-TGCGCTTGCAGAGATTAAAA-3′, (reverse) 5′-CTGCCGTACAACTCCAGTGA-3′; β-actin (forward) 5′-GCGTGACATCAAAGAGAAG-3, (reverse) 5′-ACTGTGTTGGCATAGAGG-3′.
PKC assay
For this assay, HK2 cells were deprived of serum and PKC activity then measured in immunoprecipitates with anti PKCα or PKCβ antibodies, as previously described [28].
Statistical methods
Data were analysed with Statview software (Abacus Concepts, Piscataway, NJ, USA) by one-factor analysis of variance. p values of less than 0.05 were considered statistically significant.
Results
Kidney phenotype in PEDTg mice
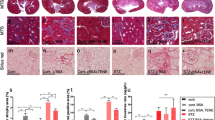
PED was present at comparable levels in renal cortex and medulla of C57/BL6 mice (Fig. 1a). A 4.5-fold overproduction was detected in PEDTg mice compared with their wild-type littermates. In 6- and 9-month-old PEDTg mice, urine volume was increased by about two- and threefold, respectively, compared with controls, accompanied by a similarly sized increase of daily urinary albumin excretion (Table 1). No significant differences were detected in younger animals (3 months). In addition, at 9 months, plasma creatinine levels were significantly elevated only in PEDTg. Specific gravity, urinary pH and glucose and kidney weight, as well as food and water intake, blood glucose, HbA1c, systolic and diastolic blood pressure, and heart rate did not show significant differences at 3, 6 and 9 months of age. Moreover, consistent with previous reports [25], fasting insulin, NEFA and triacylglycerol were higher in PEDTg than in wild-type mice, independently of age. In 9-month-old PEDTg mice, histological examination of the kidney, followed by morphometrical analysis, revealed a 2.3-fold increase of mesangial area compared with their wild-type littermates (Fig. 1b). Again, no change was detected in 3- and 6-month-old mice (data not shown). Thus, as well as deranging glucose tolerance [25], overproduction of PED induces age-dependent mesangial expansion and affects renal function in transgenic mice.
PED levels, mesangial expansion and TGF-β1 levels/expression in PEDTg. a Cortex (C) and medulla (M) of the kidneys from wild-type (WT) and PEDTg were dissected, solubilised and western blotted with anti-PED antibodies. Blots were revealed by ECL and autoradiography, and quantified by laser densitometry, as shown in bar graph. Values were normalised for actin. Each bar represents the mean ± SD of duplicate determinations in ten mice per group. b Mesangial area from 9-month-old WT and PEDTg mice was measured as described. Bars represent mean ± SD of determinations in ten mice per group. c Serum TGF-β1 levels were determined by ELISA in PEDTg (black bars) and control mice (white bars) at 3, 6 and 9 months, n = 15/group. d Ped mRNA was determined by real-time RT-PCR analysis of total RNA isolated from kidneys of the PEDTg (black bars) and control mice (white bars), using β-actin as internal standard. Each bar represents the mean ± SD of four independent experiments in each of which reactions were performed in triplicate using the pooled total RNAs obtained from seven mice per genotype. *p < 0.05, **p < 0.01, ***p < 0.001
TGF-β1 is a cytokine that has been extensively studied as a major mediator of kidney damage in diabetic nephropathy [15]. We therefore measured plasma and renal TGF-β1 levels in PEDTg animals. In 9-month-old PEDTg mice we saw a greater than twofold increase of circulating TGF-β1 levels compared with wild-type littermates (Fig. 1c). Moreover, Tgf-β1 (also known as Tgfb1) mRNA content was increased by about twofold in kidney extracts of PEDTg mice (Fig. 1d). Less evident, although significant, changes were detected in 6-month-old but not in 3-month-old PEDTg mice.
Effect of PED overexpression on TGF-β1 signalling in HK2 proximal tubular cells
To address whether PED directly regulates TGF-β1 expression in renal cells, HK2 proximal tubular cells were stably transfected with a PED full-length cDNA. Several clones were selected and characterised, of which two, namely those displaying lower (HKPED1) and higher (HKPED2) PED levels, were studied in detail (Fig. 2a). TGF-β1 mRNA content was increased by 1.5- and 2.1-fold, respectively in HKPED1 and HKPED2 cells (Fig. 2b), while connective tissue growth factor levels did not change (data not shown). Moreover, TGF-β1 levels were increased by 2.6- and 3.2-fold in the culture media of HKPED1 and HKPED2, respectively, compared with the parental HK2 cells (Fig. 2c). In addition, the amount of phosphorylated SMAD2/3 in the transfected clones was higher than in control cells, with no change in the cellular content of SMAD2 and SMAD3 proteins (Electronic supplementary material [ESM] Fig. 1), indicating functional activation of the TGF-β1 signalling pathway. Further supporting this hypothesis, treatment of HK2 cells with 0.1 μmol/l SB431542, a TGF-β1 receptor kinase inhibitor, reduced SMAD2 and SMAD3 phosphorylation by 40% (Fig. 3a). A 50% decrease was observed in HKPED2 cells. Total SMAD2 and SMAD3 levels were unchanged following exposure of both cell types to SB431542 (Fig. 3a). Results consistent with the above were obtained in HKPED1 cells (data not shown).
Effect of PED overexpression on TGF-β1 in HK2 cells. HK2 cells were stably transfected with PED cDNA. HKPED1 and HKPED2 represent two different clones. Cell lysates were analysed by SDS-PAGE followed by blotting (a) with PED or actin antibodies. Alternatively (b), the abundance of TGF-β1 mRNA was determined by real-time RT-PCR analysis, using β-actin as internal standard. c TGF-β1 levels were measured in the culture medium by ELISA assay. Bars represent the means ± SD of triplicate measurements in four independent experiments. *p < 0.05, **p < 0.01
Effect of PED overexpression and of PLD, PKC and TGF-β1 inhibition on fibronectin production in HK2 cells. a HK2 and HK2PED2 cells were incubated with SB431542 (0.1 μmol/l). Total SMAD and actin levels, and SMAD2/3 phosphorylation were measured as described. A representative autoradiograph of four independent experiments is shown, with densitometric analysis of pSMAD2/3:SMAD2 ratio, normalised for actin levels, in bar graph. Values are mean ± SD, **p < 0.01. b Culture media with different glucose concentrations (white bars, 5 mmol/l; black bars 11.2 mmol/l; grey bars, 30 mmol/l) from control and Ped overexpressing cells were collected and subjected to western blot with fibronectin antibodies. Bands were quantified by laser densitometry and normalised for actin of the corresponding cultured cells. Bars represent means ± SD of three independent experiments in triplicate. *p < 0.05, **p < 0.01. c Culture media from HK2 and HK2PED2 cells or (d) from murine tubular epithelial cells isolated from wild-type (WT) and PEDTg mice were collected, immunoblotted with fibronectin antibody. Bands were quantified by laser densitometry and normalised for actin of the corresponding cultured cells. Bars represent the mean ± SD of duplicate determinations in five independent experiments. **p < 0.01; † p < 0.01 for differences between HK2PED2 and HK2 cells
Effect of PED overexpression on fibronectin production in HK2 cells
TGF-β1 regulates the synthesis of key extracellular matrix proteins, including fibronectin, laminin, and collagen I and IV [14, 16, 18, 19]. Thus, consistent with the augmented TGF-β1 production, 1.5- and 1.9-fold increased levels of fibronectin were detected, independently of glucose concentration (5.0, 11.2 and 30.0 mmol/l), in the media of HKPED1 and HKPED2, respectively, compared with control cells (Fig. 3b). Slight, though not statistically significant, increases of laminin and both collagen I and IV were also observed (data not shown).
We have previously shown that overproduction of PED in cultured cells leads to activation of the PLD/PKC pathway [23, 27]. To study the possible involvement of PLD and PKC as potential mediators of PED-induced fibronectin production, wild-type and HKPED2 cells were incubated for 15 min with the PLD inhibitor 2-butanol (0.3% vol./vol.) and with the classical PKC inhibitor bisindolylmaleimide (BIM) (100 nmol/l) or the specific PKCβ inhibitor LY379196 (50 nmol/l). At the concentrations used, these compounds did not inhibit PKCα (ESM Fig. 2a) and PKCβ (ESM Fig. 2b) activity in parental HK2 cells. HKPED2 cells displayed higher levels of PLD (data not shown), PKCα (ESM Fig. 2a) and PKCβ (ESM Fig. 2b) activity. In these cells, PKCα activity was unchanged by LY379196 and reduced by 30% and 35% following exposure to 2-butanol and BIM, respectively. PKCβ activity was decreased by 30%, 32% and 60% following exposure to 2-butanol, BIM and LY379196 respectively. Finally, no change of PKCα (ESM Fig. 2a) and PKCβ (ESM Fig. 2b) activity was induced by treatment with SB431542, either in HK2 or in HKPED2 cells, indicating that TGF-β1 was not upstream to PKC activation.
In parallel with the unmodified PKC activity, neither PLD nor PKC inhibitors reduced fibronectin production by native HK2 cells (Fig. 3c). In contrast, exposure of HKPED2 cells for 15 min to 0.3% 2-butanol, 100 nmol/l BIM and 50 nmol/l LY379196 decreased fibronectin by 37%, 22% and 55%, respectively. A similar effect was also observed following treatment with SB431542 for 15 min, which reduced fibronectin levels by 40% in HKPED2 cells, without affecting control cells (Fig. 3c). To confirm these data, we also measured fibronectin production in primary cultures from the kidneys of wild-type and PEDTg mice. As shown in Fig. 3d, exposure to 2-butanol, BIM, LY379196 and SB431542, respectively reduced the levels of fibronectin by 47%, 38%, 38% and 50% in PEDTg-derived cells only. Thus, these data indicate that PLD, PKCβ and TGF-β1 signalling are required for regulation of fibronectin release by cells overexpressing PED.
Effect of PLD and PKC inhibition on TGF-β1 signalling in HK2 cells
We then measured TGF-β1 production and function in parental HK2 and in HKPED2 cells following PLD and PKC inhibition. 2-Butanol and BIM had no significant effect in the HK2 cells, but reduced TGF-β1 levels in HKPED2 cells by 45% and 40%, respectively (Fig. 4a). Interestingly, the selective block of PKCβ with LY379196 led to 65% reduction of TGF-β1 levels in HKPED2 cells, while being ineffective in HK2 cells. The effect of PKCβ inhibition was also well detectable in terms of reduction of SMAD2 phosphorylation, as well as of SMAD3 (data not shown), with an almost complete block following treatment with LY379196 of HKPED2 cells (Fig. 4b).
Effect of PLD and PKC on TGF-β1 signalling in HK2 cells overexpressing PED. HK2 and HK2PED2 cells were incubated with 2-butanol (But), BIM, LY379196 or SB431542 for 15 min. a TGF-β1 levels were measured in the culture media by ELISA assay. Bars represent means ± SD of triplicate measurements in four independent experiments. b Cells were solubilised and lysates analysed by SDS-PAGE and western blotted with total Smad2 or Smad3, p-Smad2/3 and actin antibodies. The autoradiograph shown is representative of four independent experiments. Densitometric analysis of pSMAD2/3:SMAD2 ratio is shown in bar graph, with values as mean ± SD. *p < 0.05, **p < 0.01; †p < 0.05 for differences between HK2PED2 and HK2
Role of TGF-β1 signalling in PED-mediated fibronectin production
Next, HK2 cells were transiently transfected with SMAD2- or SMAD3- specific phosphorothioate antisense oligonucleotides (SMAD2 AS and SMAD3 AS) to block production of the two proteins. SMAD2 AS and SMAD3 AS caused a > 70% reduction of SMAD2 and -3 compared with scrambled oligonucleotides, both in wild-type and in HKPED2 cells (ESM Fig. 3a). Specific depletion of the SMAD2 and SMAD3 isoforms was followed by a 30% and 35% fibronectin reduction in HK2 cells and by 60% and 65% reductions in HKPED2 clones, compared with the respective cell clone treated with scrambled control oligonucleotides (ESM Fig. 3b). Similar results were obtained in HKPED1 cells (data not shown). Thus, overproduction of PED upregulated fibronectin production through a TGF-β1/SMAD2/3-dependent pathway.
PED depletion decreases TGF-β1 signalling and fibronectin production
To assess whether endogenous PED controls TGF-β1 and fibronectin production, HK2 cells were transiently transfected with a PED-specific phosphorothioate antisense oligonucleotide (PED-AS). PED-AS treatment induced a 65% PED depletion compared with a scrambled oligonucleotide (PED-SC) (Fig. 5a).
Role of PED depletion in TGF-β1 signalling. HK2 cells were transiently transfected with PED antisense (PED-AS) or scrambled (PED-SC) oligonucleotides. a PED was detected by western blot, as previously described. The autoradiograph is representative of four independent experiments. b TGF-β1 was measured by ELISA. Bars represent means ± SD of duplicate measurements in four independent experiments. c Total SMAD2 and SMAD3 levels, and SMAD2/3 phosphorylation were detected by western blot, with densitometric analysis (bar graph) of pSMAD2/3:SMAD2 ratio. Values are mean ± SD. d Fibronectin from the culture media of the cells was analysed by western blot and quantified by bar graph, mean ± SD of duplicate determinations in four independent experiments. **p < 0.01
TGF-β1 levels were about 60% lower in PED-AS cells than in the controls (Fig. 5b) and, in parallel, SMAD2, as well as SMAD3 (data not shown) phosphorylation was decreased by about 50% in the presence of the antisense (Fig. 5c). However, total SMAD protein levels were unchanged. Consistently, fibronectin secretion was reduced by about 50% in cells treated with PED-AS compared with those treated with PED-SC (Fig. 5d). Consistent results were obtained in Ped-KO [32] (Fig. 6a). In spite of normal glucose levels (Table 2), Ped gene ablation coincided with a 65% and 60% decrease of TGF-β1 plasma levels and renal mRNA content, respectively, compared with control mice (Fig. 6b, c). SMAD2 phosphorylation was also reduced by 40% in kidney extracts of Ped-KO (Fig. 6d). SMAD3 phosphorylation also displayed a similar decrease (data not shown).
Determination of serum and renal TGF-β1 levels, and SMAD2/3 phosphorylation in Ped-KO. a Kidneys from wild-type and Ped-KO were dissected, solubilised and western blotted with anti-PED or actin antibodies. Blots were revealed by ECL and autoradiography (representative autoradiograph of four independent experiments). b Serum TGF-β1 levels were determined in Ped-KO and control mice (n = 11 per group) by ELISA assay. Bars are the mean ± SD of duplicate determinations. ***p < 0.001. c The abundance of Ped mRNA was determined by real-time RT-PCR analysis of total RNA isolated from kidneys of the KO and control mice, using β-actin as internal standard. Bars represent the mean ± SD of four independent experiments in each of which reactions were performed in triplicate using the pooled total RNAs obtained from five mice per genotype. ***p < 0.001. d Tissue lysates from kidneys of the KO and control mice (n = 5 per group) were pooled and analysed by SDS-PAGE followed by blotting with total SMAD2 or SMAD3, p-SMAD2/3 and actin antibodies. The autoradiograph is representative of three independent experiments, with densitometric analysis of pSMAD2/3:SMAD2 ratio in bar graph, mean ± SD. **p < 0.01
Correlation between individual levels of PED and plasma TGFβ in humans
We had analysed plasma TGF-β1 levels and PED protein abundance in PBLs in 95 type 2 diabetic patients matched for age, BMI, waist circumference, systolic and diastolic BP, and fasting HDL-cholesterol [24]. As shown in Fig. 7, there was a positive correlation between the individual levels of PED and plasma TGF-β1.
Correlation between PED levels from PBLs and plasma TGF-β1 levels in type 2 diabetic patients. PED protein levels were analysed by western blot in PBLs from 95 type 2 diabetic patients and bands were quantified by laser densitometry as described. Plasma TGF-β1 levels were measured by ELISA. Linear regression analysis revealed a positive correlation between PED and TGF-β1 levels (r = 0.593, p < 0.001)
Discussion
The molecular mechanisms responsible for the onset and/or progression of renal complications of diabetes mellitus are still poorly understood. While hyperglycaemia plays a crucial role [2–5], other environmental conditions as well as specific genetic backgrounds may strongly contribute to the clinical appearance of the disorder, either as protective or as predisposing factors [39]. A further complicating element for understanding the molecular determinants is the lack of animal models fully representative of human diabetic nephropathy [40]. We have previously generated a transgenic mouse ubiquitously expressing high levels of PED, a gene whose expression is elevated in a large proportion of individuals with type 2 diabetes and in their first-degree relatives [21, 24]. In mice, PED overproduction impairs glucose tolerance by affecting insulin action and secretion [25, 41], and the animals develop overt diabetes following administration of high-fat diets [25].
Here we show that PEDTg mice also displayed mesangial expansion, and mildly elevated plasma creatinine levels and urinary albumin excretion between 6 and 9 months of age, in the absence of frank hyperglycaemia. Indeed, blood glucose, systolic and diastolic blood pressure, heart rate and HbA1c did not significantly change in wild-type and PEDTg mice, when early signs of renal disorder were found. The renal functional changes were paralleled by elevation of TGFβ1 levels, a cytokine which has been commonly found elevated in patients with diabetic nephropathy [11–15]. However, none of these abnormalities was detectable in younger littermates, suggesting that while PED overproduction represents an early defect, it may also play a role in the slow progression toward renal damage.
Since glucose tolerance of these animals is significantly reduced, and plasma triacylglycerol and NEFA are increased [25], we cannot exclude the possibility that the renal abnormalities observed in PEDTg mice could be linked to the metabolic phenotype. Consistent with this hypothesis, individuals with impaired glucose tolerance display a higher risk of developing nephropathy [42], and microalbuminuria often precedes the onset of diabetes [43]. Nevertheless, studies in HK2 human proximal tubular cells and in primary cultures from the kidneys have shown that selective overexpression of PED in kidney cells may, at least in part, be directly responsible for renal derangement. In this regard, forced expression of PED increased the production of extracellular matrix (ECM) proteins, whose accumulation is a hallmark of mesangial expansion [17]. In addition, these changes occurred independently of glucose concentration in the extracellular media (Fig. 3b) and of insulin treatment (data not shown), indicating that elevation of PED levels is sufficient to impair ECM production.
The potential mechanisms leading to hyperproduction of ECM proteins are likely to involve hyperactivation of PKCβ and the TGF-β1 signalling pathways. PED binds PLD, increasing its intracellular stability and leading to the accumulation of diacylglycerol [23, 27]. This, in turn, determines abnormal activation of conventional PKC isoforms, including PKCα and PKCβ. Increased PKCβ activity may account, at least in part, for the renal phenotype observed in PEDTg mice. In HK2 cells, inhibition of PKCβ largely reversed the effect of PED overexpression. In particular, the increase in TGF-β1 mRNA levels and signalling caused by PED in HK2 cells was almost completely blocked by the PKCβ inhibitor. Interestingly, both PKCβ hyperactivity and elevated TGF-β1 levels are commonly found in diabetic nephropathy, both in vitro and in vivo [44–46]. In several experimental and clinical studies, inhibition of PKC (LY333531) has been shown to delay or halt the progression of diabetic complications [47]. Part of the beneficial effect of PKC inhibition is due to the consequent downregulation of TGF-β1 [48]. Consistently, higher circulating levels of TGF-β1 have been detected in PEDTg mice and are paralleled by increased Tgf-β1 mRNA content in kidney extracts. However, the question of whether other cell types contribute to the increase of TGF-β1 content in the bloodstream is currently not resolved and under investigation in our laboratory.
Inhibition of PLD and PKCβ, as well as of TGF-β1 receptor activity, or genetic silencing of SMAD2 and SMAD3 decreased production of fibronectin. Thus, PED overexpression may lead to increased PKCβ activity, which enhances expression of TGF-β1, which in turn upregulates fibronectin production. Further support of this hypothesis was seen in the fact that inhibition of PED by antisense oligonucleotides in HK2 cells was paralleled by reduced PKCβ activity and TGF-β1 expression and signalling, as well as by decreased fibronectin production. Similarly to PED-depleted cells, kidney and total TGF-β1 levels and PKCβ activity were reduced in animals bearing the complete ablation of Ped gene, indicating a cause–effect relationship.
It remains unknown whether PED overproduction is involved in diabetic nephropathy in humans. However, in a cohort of patients with type 2 diabetes [24], we found a positive correlation between levels of PED measured in PBLs and plasma TGFβ1 levels. It could therefore be inferred that high expression levels of PED deranges renal function in a subset of diabetic individuals, thereby facilitating the onset and/or progression of diabetic nephropathy.
The mechanism leading to the increase of PED cellular levels are only partially known and may be due to altered transcriptional control [49] or to post-translational modifications [50]. The contribution of genetic variations and epigenetic modifications is currently under investigation in our laboratory.
Thus, high expression levels of PED determine an increase of PKCβ activity and of TGF-β1 expression in cellular and animal models, as well as in humans. They therefore may represent an early abnormality, contributing to the derangement of ECM deposition and to progression toward nephropathy.
Abbreviations
- BIM:
-
Bisindolylmaleimide
- ECL:
-
Enhanced chemiluminescence
- ECM:
-
Extracellular matrix
- HK2 cells:
-
Human kidney 2 cells
- PBL:
-
Peripheral blood leucocyte
- PED:
-
Phosphoprotein enriched in diabetes/phosphoprotein enriched in astrocytes-15
- Ped-KO:
-
Ped/Pea-15 knockout mice
- PEDTg:
-
Transgenic mice overexpressing PED
- PKC:
-
Protein kinase C
- PLD:
-
Phospholipase D
- SMAD:
-
SMAD family member
References
Wolf G (2004) New insights into the pathophysiology of diabetic nephropathy: from haemodynamics to molecular pathology. Eur J Clin Invest 34:785–796
The Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 329:977–986
UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352:837–853
Members of the ADVANCE collaborative group (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358:2560–2572
Bilous R (2008) Microvascular disease: what does the UKPDS tell us about diabetic nephropathy? Diabet Med 25(Suppl 2):25–29
Fogarty DG, Hanna LS, Wantman M, Warram JH, Krolewski AS, Rich SS (2000) Segregation analysis of urinary albumin excretion in families with type 2 diabetes. Diabetes 49:1057–1063
Imperatore G, Knowler WC, Pettitt DJ, Kobes S, Bennett PH, Hanson RL (2000) Segregation analysis of diabetic nephropathy in Pima Indians. Diabetes 49:1049–1056
Boright AP, Paterson AD, Mirea L et al (2005) Genetic variation at the ACE gene is associated with persistent microalbuminuria and severe nephropathy in type 1 diabetes: the DCCT/EDIC Genetics Study. Diabetes 54:1238–1244
Flyvbjerg A (2000) Putative pathophysiological role of growth factors and cytokines in experimental diabetic kidney disease. Diabetologia 43:1205–1223
Wolf G (2003) Growth factors and the development of diabetic nephropathy. Curr Diab Rep 3:485–490
Azar ST, Salti I, Zantout MS, Major S (2000) Alteration in plasma transforming growth factor β in normoalbuminuric type 1 and type 2 diabetic patients. J Clin Endocrinol Metab 85:4680–4682
Pfeiffer A, Middleberg-Bisping K, Drewes C, Schatz H (1996) Elevated plasma levels of transforming growth factor β-1 in NIDDM. Diabetes Care 18:1113–1117
Ziyadeh FN (2004) Mediators of diabetic renal disease the case for TGF-beta as the major mediator. J Am Soc Nephrol 15(Suppl 1):S55–S57
Wang W, Koka V, Lan HY (2005) Transforming growth factor-beta and Smad signalling in kidney diseases. Nephrology 10:48–56
Sharma K, Ziyadeh FN (1995) Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediator. Diabetes 44:1139–1146
Ha H, Yu MR, Lee HB (2001) High glucose-induced PKC activation mediates TGF-beta 1 and fibronectin synthesis by peritoneal mesothelial cells. Kidney Int 59:463–470
Bloomgarden ZT (2005) Diabetic nephropathy. Diabetes Care 28:745–751
Cheng J, Grande JP (2002) Transforming growth factor-beta signal transduction and progressive renal disease. Exp Biol Med 227:943–956
Ten Dijke P, Hill CS (2004) New insights into TGF-beta-Smad signalling. Trends Biochem Sci 29:265–273
Araujo H, Danziger N, Cordier J, Glowinski J, Chneiweiss H (1993) Characterization of PEA-15 a major substrate for protein kinase C in astrocytes. J Biol Chem 268:5911–5920
Condorelli G, Vigliotta G, Iavarone C et al (1998) PED/PEA-15 gene controls glucose transport and is overexpressed in type 2 diabetes mellitus. EMBO J 7:3858–3866
Condorelli G, Trencia A, Vigliotta G et al (2002) Multiple members of the mitogen-activated protein kinase family are necessary for PED/PEA-15 anti-apoptotic function. J Biol Chem 277:11013–11018
Condorelli G, Vigliotta G, Trencia A et al (2001) Protein kinase C (PKC)- α activation inhibits PKC- ζ and mediates the action of PED/PEA-15 on glucose transport in the L6 skeletal muscle cells. Diabetes 50:1244–1252
Valentino R, Lupoli G, Raciti GA et al (2006) The PEA15 gene is overexpressed and related to insulin resistance in healthy first-degree relatives of patients with type 2 diabetes. Diabetologia 49:3058–3066
Vigliotta G, Miele C, Santopietro S et al (2004) Overexpression of the ped/pea-15 gene causes diabetes by impairing glucose-stimulated insulin secretion in addition to insulin action. Mol Cell Biol 24:5005–5015
Zhang Y, Redina O, Altshuller YM et al (2000) Regulation of expression of phospholipase D1 and D2 by PEA-15, a novel protein that interacts with them. J Biol Chem 275:35224–35232
Viparelli F, Cassese A, Doti N et al (2008) Targeting of PED/PEA-15 molecular interaction with phospholipase D1 enhances insulin sensitivity in skeletal muscle cells. J Biol Chem 283:21769–21778
Caruso M, Miele C, Oriente F et al (1999) In L6 skeletal muscle cells, glucose induces cytosolic translocation of protein kinase C alfa and transactivates the insulin receptor kinase. J Biol Chem 274:28637–28644
Li Y, Soos TJ, Li X et al (2004) Protein kinase C theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101). J Biol Chem 27:45304–45307
Ishizuka T, Kajita K, Natsume Y et al (2004) Protein kinase C (PKC) beta modulates serine phosphorylation of insulin receptor substrate-1 (IRS-1)—effect of overexpression of PKCbeta on insulin signal transduction. Endocr Res 30:287–299
Nakajima K, Yamauchi K, Shigematsu S et al (2000) Selective attenuation of metabolic branch of insulin receptor down-signaling by high glucose in a hepatoma cell line HepG2 cells. J Biol Chem 275:20880–20886
Kitsberg D, Formstecher E, Fauquet M et al (1999) Knock-out of the neural death effector domain protein PEA-15 demonstrates that its expression protects astrocytes from TNFalpha-induced apoptosis. J Neurosci 19:8244–8251
Ciccarelli M, Santulli G, Campanile A et al (2008) Endothelial alpha1-adrenoreceptors regulate neoangiogenesis. Br J Pharmacol 153:936–946
Kasahara M, Mukoyama M, Sugawara A et al (2000) Ameliorated glomerular injury in mice overexpressing brain natriuretic peptide with renal ablation. J Am Soc Nephrol 11:1691–1701
Ziyadeh FN, Hoffman BB, Han DC et al (2000) Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci U S A 97:8015–8020
Terryn S, Jouret F, Vandenabeele F et al (2007) A primary culture of mouse proximal tubular cells, established on collagen-coated membranes. Am J Physiol Renal Physiol 293:F476–F485
Hao C, Beguinot F, Condorelli G et al (2001) Induction and intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apoptosis in human malignant glioma cells. Cancer Res 61:1162–1170
Miele C, Caruso M, Calleja V et al (1999) Differential role of insulin receptor substrate (IRS)-1 and IRS-2 in L6 skeletal muscle cells expressing the Arg1152 → Gln insulin receptor. J Biol Chem 274:3094–3102
Granier C, Makni K, Molina L, Jardin-Watelet B, Ayadi H, Jarraya F (2008) Gene and protein markers of diabetic nephropathy. Nephrol Dial Transplant 23:792–799
Breyer MD, Böttinger E, Brosius FC 3rd et al (2004) AMDCC. Mouse models of diabetic nephropathy. J Am Soc Nephrol 16:27–45
Miele C, Raciti GA, Cassese A et al (2007) PED/PEA-15 regulates glucose-induced insulin secretion by restraining potassium channel expression in pancreatic beta-cells. Diabetes 56:622–633
Singleton JR, Smith AG, Russell JW, Feldman EL (2003) Microvascular complications of impaired glucose tolerance. Diabetes 52:2867–2873
Mykkanen L, Haffner SM, Kuusisto J, Pyorala K, Laakso M (1994) Microalbuminuria precedes the development of NIDDM. Diabetes 43:552–557
Koya D, Jirousek MR, Lin YW, Ishii H, Kuboki K, King GL (1997) Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J Clin Invest 100:115–126
Meier M, Park JK, Overheu D et al (2007) Deletion of protein kinase C-beta isoform in vivo reduces renal hypertrophy but not albuminuria in the streptozotocin-induced diabetic mouse model. Diabetes 56:346–354
Kelly DJ, Zhang Y, Hepper C et al (2003) Protein kinase C beta inhibition attenuates the progression of experimental diabetic nephropathy in the presence of continued hypertension. Diabetes 52:512–518
Das Evcimen N, King GL (2007) The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res 55:498–510
Koya D, Haneda M, Nakagawa H et al (2000) Amelioration of accelerated diabetic mesangial expansion by treatment with a PKCbeta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J 14:439–447
Ungaro P, Teperino R, Mirra P et al (2008) Molecular cloning and characterization of the human PED/PEA-15 gene promoter reveal antagonistic regulation by hepatocyte nuclear factor 4alpha and chicken ovalbumin upstream promoter transcription factor II. J Biol Chem 283:30970–30979
Perfetti A, Oriente F, Iovino S et al (2007) Phorbol esters induce intracellular accumulation of the anti-apoptotic protein PED/PEA-15 by preventing ubiquitinylation and proteasomal degradation. J Biol Chem 282:8648–8657
Acknowledgements
This study was supported by the European Community’s FP6 EUGENE2 (LSHM-CT-2004-512013) and PREPOBEDIA (201681) projects, the European Foundation for the Study of Diabetes and grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC) and the Ministero dell’Università e della Ricerca Scientifica (PRIN and FIRB). The financial support of Telethon–Italy is also gratefully acknowledged.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Oriente and S. Iovino contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
SMAD phosphorylation in HK2 cells transfected with PED. HK2, HK2PED1 and HK2PED2 cells were analysed by SDS-PAGE followed by blotting with total SMAD2 or SMAD3, pSMAD2/3 and actin antibodies. The autoradiograph is representative of four independent experiments, quantified in bar graph by densitometric analysis of pSMAD2/3:SMAD2 ratio, normalised for actin levels; values mean ± SD. *p < 0.05, **p < 0.01 (PDF 36 kb)
ESM Fig. 2
Effect of PLD, PKC and TGF-β inhibition on specific PKC isoform activities and fibronectin production in HK2 cells. HK2 and HK2PED2 cells were incubated with 2-butanol (But) (0.3%), BIM (100 nmol/l), LY379196 (50 nmol/l) or SB431542 (0.1 μmol/l) for 15 min. Cells were solubilised and immunoprecipitated with isoform-specific PKC antibodies. PKCα (a) and PKCβ (b) activities were then assayed in the immunoprecipitates. Bars represent the mean ± SD of duplicate determinations in four independent experiments. *p < 0.05, **p < 0.01; † p < 0.05, †† p < 0.01 for differences between HK2PED2 and HK2 cells (PDF 59 kb)
ESM Fig. 3
Effect of SMAD2 and SMAD3 silencing on fibronectin production in HK2 cells overexpressing PED. HK2 and HK2PED2 cells were transfected with SMAD2 and SMAD3 antisense (Sm2-AS and Sm3-AS, respectively) or scrambled (Sm2-SC and Sm3-SC, respectively) oligonucleotides. Total SMAD levels and SMAD2/3 phosphorylation (a), and fibronectin levels (b) were measured as described above. Autoradiographs (a, b) are representative of four independent experiments. Bars (b) represent the mean ± SD of duplicate determinations in four independent experiments. *p < 0.05, **p < 0.01; †† p < 0.01 for differences between HK2PED2 and HK2 (PDF 44 kb)
Rights and permissions
About this article
Cite this article
Oriente, F., Iovino, S., Cassese, A. et al. Overproduction of phosphoprotein enriched in diabetes (PED) induces mesangial expansion and upregulates protein kinase C-β activity and TGF-β1 expression. Diabetologia 52, 2642–2652 (2009). https://doi.org/10.1007/s00125-009-1528-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-009-1528-z