Abstract
Aims/hypothesis
Diabetes is known to increase mortality rate, but the degree to which mild hyperglycaemia may be associated with the risk of death is uncertain. We examined the association between HbA1c measured in stored erythrocytes and mortality rate in women with and without diabetes.
Methods
We conducted a cohort study of 27,210 women ≥ 45 years old with no history of cardiovascular disease or cancer who participated in the Women’s Health Study, a randomised trial of vitamin E and aspirin.
Results
Over a median of 10 years of follow-up, 706 women died. Proportional hazards models adjusted for age, smoking, hypertension, blood lipids, exercise, postmenopausal hormone use, multivitamin use and C-reactive protein were used to estimate the relative risk of mortality. Among women without a diagnosis of diabetes and HbA1c <5.60%, those in the top quintile (HbA1c 5.19–5.59%) had a relative risk of mortality of 1.28 (95% CI 0.98–1.69, p value for linear trend = 0.14) compared with those with HbA1c 2.27–4.79%. Women with HbA1c 5.60–5.99% and no diagnosis of diabetes had a 54% increased risk of mortality (95% CI 1–136%) compared with those with HbA1c 2.27–4.79%. HbA1c was significantly associated with mortality across the range 4.50–7.00% (p value for linear trend = 0.02); a test of deviation from linearity was not statistically significant (p = 0.67). Diabetic women had more than twice the mortality risk of non-diabetic women.
Conclusions/interpretation
This study provides further evidence that chronic mild hyperglycaemia, even in the absence of diagnosed diabetes, is associated with increased risk of mortality.
ClinicalTrials.gov ID no.: NCT00000479
Similar content being viewed by others
Introduction
Diabetes has long been recognised as a strong risk factor for mortality, particularly from cardiovascular causes [1]. While the diagnostic criteria for type 2 diabetes are based on a threshold in the risk of microvascular complications [2], mounting evidence suggests that blood glucose in the high-normal or prediabetic range is associated with increased cardiovascular disease [3–9] and cancer [10–15], though results have not been consistent [16–22]. HbA1c is commonly used to monitor glycaemic control among people with diabetes mellitus, but it may also be useful to assess exposure to glucose among those without diabetes because it is an integrated measure of glycaemia over the preceding several weeks and does not require a fasting blood sample. Widely accepted guidelines for diagnosis of diabetes based on HbA1c have not been developed, but HbA1c has been associated with an increased risk of mortality among people with and without diabetes in several epidemiological studies [5, 7, 23, 24]. It is not known whether there is a threshold in the relationship between HbA1c and mortality rate, similar to the microvascular complications of hyperglycaemia [2], or whether the association is linear across the range, similar to the cardiovascular effects [3]. We examined the association between HbA1c measured in stored erythrocytes and mortality among 27,210 participants in the Women’s Health Study after 10 years of follow-up, with attention to potential non-linearity.
Methods
Study participants
The Women’s Health Study was a 2 × 2 factorial, randomised, placebo-controlled trial of low-dose aspirin and vitamin E for the primary prevention of cardiovascular disease and cancer [25–27]. The study has been described in detail previously [22, 27, 28]. Briefly, 39,876 female health professionals aged 45 years and older were enrolled between November 1992 and July 1995. Eligible women had no history of cardiovascular disease or cancer (other than non-melanoma skin cancer) and either did not plan to become pregnant or were postmenopausal. Participants provided baseline information about demographic, behavioural and lifestyle factors, medical history including diagnosis of diabetes, height and weight.
Baseline screening tests for diabetes were not performed as part of the study so diabetes was potentially underdiagnosed. Because we were interested in the association of HbA1c with mortality rate in women without diabetes, we initially classified apparently non-diabetic women by HbA1c <5.60% or ≥5.60%, the 94th percentile of HbA1c among Women’s Health Study participants. This cut-off point was chosen a priori to distinguish women with potential undiagnosed diabetes based on the maximum sensitivity and specificity of HbA1c as a screening test for diabetes in the Third National Health and Nutrition Survey (1988–1994, USA). In that nationally representative sample, HbA1c ≥5.60% had a sensitivity of 83.4% and a specificity of 84.4% as a screening test, compared with fasting blood glucose ≥7 mmol/l, the diagnostic criterion recommended by the American Diabetes Association [2, 29]. In our study of the 27,210 women who provided a usable blood sample and information on height and weight, 661 women reported a diagnosis of diabetes and 1,594 had HbA1c ≥ 5.60%, including 1,024 with no self-reported diagnosis of diabetes. We classified women with HbA1c <5.60% and no diagnosis of diabetes by quintiles of HbA1c. We divided women with elevated HbA1c and no diagnosis of diabetes into two groups: those with HbA1c 5.60–5.99 and ≥6.00% (including individuals with probable undiagnosed diabetes). Clinical laboratories often consider HbA1c ≥ 6.00% abnormal [30], and this level of HbA1c has high specificity as a screening test for diabetes [29]. We additionally classified women who reported a diagnosis of diabetes at baseline by HbA1c <7.00 or ≥7.00%, based on recommended targets for glycaemic control in people with diabetes [31].
The institutional review board of Brigham and Women’s Hospital approved the Women’s Health Study, and all participants provided written informed consent.
Blood collection and laboratory analysis
Before beginning study medications, participants received a blood collection kit, which included collection tubes, a cooling pack and a completed courier air bill. Participants had their blood drawn and sent the samples to the laboratory by overnight courier. After processing, the samples were stored in liquid nitrogen until thawing for the analysis of HbA1c (packed erythrocytes, turbidimetric immunoinhibition assay, day-to-day variability 3.6 and 3.8% at levels of 5.2 and 8.8%, respectively), HDL-cholesterol (enzymatic colorimetric assay, day-to-day variability 2.0 and 2.7% at concentrations of 0.91 and 1.42 mmol/l, respectively), LDL-cholesterol (direct assay, day-to-day variability 3.0, 2.3 and 2.2% at concentrations of 2.33, 2.75 and 3.34 mmol/l, respectively), triacylglycerol (enzymatic assay, day-to-day variability 1.8 and 1.7% at concentrations of 0.95 and 2.28 mmol/l, respectively) and high-sensitivity C-reactive protein (hsCRP; immunoturbidimetric assay, day-to-day variability of 2.8, 1.6 and 1.1% at concentrations of 0.91, 3.07 and 13.38 mg/l, respectively). Biomarkers were analysed using a Hitachi 917 analyser (Roche Diagnostics, Indianapolis, IN, USA) and reagents from Roche Diagnostics (HbA1c, LDL-cholesterol, HDL-cholesterol and triacylglycerol) or Denka Seiken (Niigata, Japan; hsCRP). The HbA1c assay has been approved for clinical use by the United States Food and Drug Administration and is certified by the National Glycohemoglobin Standardization Program. HbA1c has been shown to be stable for approximately 1 week at 4°C and in the long term below −70°C [30, 32, 33].
Follow-up and ascertainment of mortality rate
Participants were followed from study entry until death or 31 March 2004 with yearly mailed questionnaires regarding changes in health status. Family members or postal authorities reported most deaths. Other deaths were ascertained using the National Death Index. Follow-up records on mortality were 99.4% complete. If written consent was provided, physicians reviewed medical records to determine the cause of death. In addition to total mortality rate, we evaluated three composite causes of death: (1) cardiovascular deaths, including ischaemic heart disease, acute myocardial infarction, cerebrovascular death, sudden death and death due to other cardiovascular causes; (2) ischaemic deaths, including ischaemic heart disease, acute myocardial infarction and ischaemic stroke; and (3) cancer deaths, including cancer at any site.
Participants were asked in each questionnaire whether they had been diagnosed with diabetes during the previous year. More than 90% of self-reported diabetes cases were confirmed using American Diabetes Association criteria by a follow-up questionnaire or telephone call or contact with the participant’s primary care physician [34].
Statistical analysis
We calculated age-standardised means or percentages of demographic, behavioural, lifestyle and biomarker values within each category of HbA1c. Among women with HbA1c <5.60%, we estimated the relative risk (RR) of mortality using Cox proportional hazard models adjusted for age at baseline (5 year categories), strenuous exercise (rarely/never, less than one time per week, one to three times per week, four or more times per week), postmenopausal hormone use (never, past, current), multivitamin use (never, past, current), cigarette smoking (never, past, current) and BMI (<21, 21–22.9, 23–24.9, 25–26.9, 27–28.9, 29–30.9, ≥31 kg/m2), all self-reported. We constructed additional models further adjusted for blood lipids (quintile of triacylglycerol, HDL-cholesterol and LDL-cholesterol), self-reported history of hypertension (yes or no) and a biomarker of inflammation (quintile of hsCRP). We tested for linear trends by entering the median HbA1c in each quintile as a predictor in the models.
Because vitamin E and aspirin, the trial interventions, may affect blood glucose [30, 35], we examined whether the association between HbA1c and mortality differed by randomised treatment. We tested the assumption of proportional hazards by entering the product of HbA1c and the natural logarithm of time into the model. Because some chronic diseases may disrupt glucose metabolism, we performed a sensitivity analysis excluding deaths occurring in the first 2 years of follow-up. Additionally we examined whether censoring participants at the date of diagnosis of diabetes would affect the results. We calculated the RR of mortality for apparently healthy women with HbA1c 5.60–5.99% and HbA1c ≥6.00% and for women with a diagnosis of diabetes with HbA1c <7.00% and HbA1c ≥7.00% at baseline using Cox proportional hazards models adjusted for age, lifestyle factors and biological correlates of HbA1c as described above. The models included all 27,210 women; we used the lowest quintile among women with HbA1c <5.60% as the reference group.
Modelling HbA1c as a linear, continuous exposure assumes that a 1 unit increase is associated with the same risk increase across the range of HbA1c values (i.e. an increase of HbA1c from 4.50 to 5.50% is associated with the same RR as an increase from 6.00 to 7.00%). This assumption may not be accurate. We therefore examined the possibility of non-linearity in the relationship between HbA1c and mortality using penalised cubic splines [36, 37]. Penalised splines allow the relationship between exposure and outcome to vary by levels of the exposure (i.e. the RR associated with an increase of HbA1c from 4.50 to 5.50% does not have to be equal to the RR associated with an increase from 6.00 to 7.00%). This is accomplished by dividing the exposure into several categories with a small range of values and modelling the association between exposure and outcome within each category. The association is allowed to change at the boundaries of the categories at points called knots. The overall model is constrained so that the associations within adjacent categories are similar, to avoid over-fitting and so that the curves meet smoothly without sharp corners. Details of the spline analysis are as follows: the penalised spline model had eight knots with piecewise cubic functions constrained to have approximately three degrees of freedom. The model was adjusted for age and the other covariates described above. We limited the population to 25,999 women with no diagnosis of diabetes and HbA1c 4.50–7.00% to avoid modelling where data were very sparse. We also performed exploratory analysis of the association of causes of mortality with HbA1c.
Analyses were conducted using SAS version 8 (SAS Institute, Cary, NC, USA) and S-PLUS version 6.2 (Insightful Corporation, Seattle, WA, USA), and two-sided p values < 0.05 were considered statistically significant.
Results
Over a median of 10.2 years of follow-up, 706 women died (2.6% of 27,210 participants), including 608 deaths among women with no history of diabetes and HbA1c measured in stored erythrocytes <5.60%, 49 deaths among women with no history of diabetes and HbA1c ≥5.60%, and 49 deaths among women with a history of diabetes at baseline. Women with higher HbA1c tended to be older, heavier, less likely to engage in frequent strenuous exercise, more likely to smoke, and to have a less favourable lipid profile than those with lower levels (Table 1).
Among women with HbA1c <5.60%, the risk of mortality in age-adjusted analyses was 44% higher when comparing women in the highest quintile of HbA1c with those in the lowest (RR = 1.44, 95% CI 1.11–1.88, p value for linear trend = 0.02; Table 2). Adjustment for strenuous exercise, postmenopausal hormone use, multivitamins, smoking and BMI attenuated the association, though the risk was still significantly elevated in the highest quintile (RR = 1.31, 95% CI 1.00–1.72, p value for linear trend = 0.10). After further adjustment for blood lipids, hypertension and hsCRP, the association no longer reached statistical significance (RR = 1.28 comparing extreme quintiles, 95% CI 0.98–1.69, p value for linear trend = 0.14).
We did not find evidence that the RR associated with HbA1c varied over time (p value for interaction = 0.19) or by randomisation to aspirin (p value for interaction = 0.15). The association between HbA1c and mortality was slightly stronger among women randomised to vitamin E, but this was not statistically significant (p value for interaction = 0.07). Excluding deaths that occurred during the first 2 years of follow-up and censoring participants at the time of diagnosis of diabetes did not materially change results.
Women with HbA1c 5.60–5.99% and no history of diabetes had a 54% increase in risk (RR = 1.54, 95% CI 1.01–2.36) and those with HbA1c ≥6.00% had a 66% increase in risk of mortality (RR = 1.66, 95% CI 0.96–2.85) after multivariate adjustment (Table 3). Compared with apparently healthy women with HbA1c 2.27–4.79%, women with a diagnosis of diabetes and HbA1c <7.00% had a 2.3 fold increase in risk of mortality in the fully adjusted model (RR = 2.31, 95% CI 1.42–3.76). Women with diagnosed diabetes and HbA1c ≥7.00% had a RR of mortality that was somewhat greater (RR = 2.76, 95% CI 1.74–4.37), but the difference was not statistically significant (p value = 0.54).
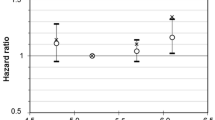
When we used penalised splines to flexibly model the association of HbA1c with mortality among apparently healthy women with HbA1c 4.50–7.00%, we found that the RR rose slowly below 5.20%, but began rising somewhat more rapidly after that point (Fig. 1). The spline model complements our finding that the risk of mortality was elevated among women with HbA1c 5.19–5.59% and further elevated among women with HbA1c ≥5.60%. However, a test for deviation from linearity was not significant (p = 0.67) and a test for a linear association was statistically significant (p = 0.02).
Association of HbA1c measured in stored erythrocytes and risk of mortality among women with no self-reported history of diabetes. The solid line represents the RR of mortality associated with HbA1c calculated from Cox proportional hazards models. Penalised cubic splines were used to flexibly model the shape of the association. Dashed lines represent the 95% confidence interval. The models were adjusted for age at baseline in 5 year categories, strenuous exercise (rarely/never, <1 time/week, 1–3 times/week, ≥4 times/week), postmenopausal hormone use (never, past, current), multivitamin use (never, past, current), smoking status (never, past, current), BMI (<21, 21–22.9, 23–24.9, 25–26.9, 27–28.9, 29–30.9, ≥31 kg/m2), self-reported history of hypertension (yes or no), and quintile of triacylglycerol, HDL-cholesterol, LDL-cholesterol and hsCRP
Most deaths among women with no history of diabetes and HbA1c <5.60% in this cohort were due to cancer (n = 354) and cardiovascular disease (n = 111). In exploratory analyses of the cause of death, the risk of cancer death was elevated in the higher two tertiles of HbA1c, but the elevation was not graded or statistically significant (Table 4). We did not observe evidence for an association between HbA1c and cardiovascular mortality in these women. Although RRs comparing extreme HbA1c tertiles were above 1 for many causes of death, the numbers of cases were small. In contrast, the risk of cardiovascular mortality was elevated in women with no history of diabetes and HbA1c ≥5.60% (RR = 1.84, 95% CI 0.96–3.54) and in women with a diagnosis of diabetes (RR = 3.60, 95% CI 1.95–6.65) after controlling for age, lifestyle, and cardiovascular risk factors. The risk of cancer mortality was non-significantly elevated in women with no history of diabetes and HbA1c ≥5.60% (RR = 1.48, 95% CI 0.90–2.43) and in women with a diagnosis of diabetes (RR = 1.60, 95% CI 0.88–2.89).
Discussion
As expected, after 10 years of follow-up in the Women’s Health Study women with a diagnosis of diabetes had a risk of mortality that was more than double that of women with HbA1c measured in stored erythrocytes <4.8%. Women without a diagnosis of diabetes but with HbA1c 5.6–6.0%, levels that are often considered normal, had a 54% increased risk of death after controlling for BMI, lifestyle factors and biological correlates of elevated blood glucose. Even apparently healthy women with HbA1c levels as low as 5.2% appeared to be at increased risk; part of the excess risk was explained by differences in BMI, lifestyle, blood lipids, hypertension and hsCRP. We did not find statistically significant deviations from linearity of the association between HbA1c and mortality rate, though the data suggest that the risk may increase more rapidly above 5.2%. Although the risk of cardiovascular mortality was increased in women with HbA1c ≥5.6% and in women with diabetes, we found no clear associations between HbA1c <5.6% and specific causes of death, possibly because of small numbers.
Compared with other populations in which the association between HbA1c and mortality has been examined, Women’s Health Study participants tended to have lower average HbA1c levels. This may be attributable to their healthy behaviours and relatively low BMI. However, the potential for degradation of the samples during shipping and storage, which could not be quantified in this study, prevents direct comparison of absolute HbA1c levels with those in studies that measured HbA1c in fresh samples.
Investigators for the EPIC (European Prospective Investigation into Cancer)–Norfolk Study reported that the risk of all-cause mortality among women increased by 28% for an increase in HbA1c of 1%, though the age-adjusted risk did not appear to be elevated below an HbA1c of 5.5% [5]. In a study combining data from the Hoorn Study and a Finnish cohort of elderly men, an increase of one standard deviation in HbA1c (∼0.67%) corresponded to a 14% increase in mortality [23]. These studies did not report threshold effects. In a cohort of elderly atomic bomb survivors, participants with HbA1c 6–6.5% had a 36% greater risk of mortality than those with HbA1c <5.5%; participants with HbA1c 5.5–6% did not have an obviously increased risk of death [24]. Investigators for the Rancho San Bernardo Study [38] and an earlier analysis of the Hoorn Study [39] did not find statistically significant associations.
HbA1c has been associated with cardiovascular mortality rate among women without diabetes in other populations [5, 24, 38]. In the present study, women with HbA1c >5.6% and no previous history of diabetes did appear to be at increased risk, but there was no association evident below this level. These results are consistent with two previous reports from the Women’s Health Study in which HbA1c was not significantly associated with incident cardiovascular disease (including fatal cases of cardiovascular disease) in women without diabetes [18, 22]. There was a suggestion that HbA1c was associated with cancer mortality, particularly colon cancer, breast cancer and lymphoma/leukaemia, but the number of cases was small. This is in contrast to the finding from the Women’s Health Study that HbA1c was not associated with incident breast or colon cancer in this population [19, 20]. However, other studies have demonstrated an increased risk of cancer incidence [10, 12–15] and mortality [11] associated with hyperglycaemia. The associations may be due to direct effects of hyperglycaemia or to other related metabolic perturbations, such as hyperinsulinaemia, that may increase the risk of cancer through direct stimulation of cancerous or precancerous cells or effects on the synthesis and bioavailability of sex hormones [40]. Insulin concentrations were not measured in this population.
The increased risk of mortality associated with HbA1c in women without self-reported diabetes may be due to their increased risk of developing diabetes during follow-up. However, when participants were censored at the time of diabetes diagnosis, the associations did not change. Although underdiagnosis is a concern, screening rates were high in this population, 85–90% of participants reporting a blood glucose test on the annual questionnaire [34].
HbA1c is not currently recommended as a diagnostic or screening test for diabetes [41] and no widely accepted diagnostic criterion for diabetes based on HbA1c has been developed. As we could not administer an oral glucose tolerance test or a fasting glucose test in this study to distinguish between participants with and without diabetes, for the primary analysis we chose a threshold HbA1c near the maximum sensitivity and specificity for the diagnosis of diabetes among participants of the Third National Health and Nutrition Examination Survey [29]. Other limitations of this study deserve mention. Approximately 97% of the participants survived the 10 years of follow-up, and the number of deaths provided low power to detect a small to moderate association between HbA1c and mortality and resulted in wide confidence intervals for the RRs, particularly for specific causes of death. We had a single measurement of HbA1c for each participant, which may have resulted in misclassification of participants due to random variability in HbA1c, potentially leading to biased results. The blood samples were shipped to the laboratory on cooling packs by the participants, where they were then stored in liquid nitrogen before analysis, which could have led to sample degradation. Changes in the samples due to shipping and storage could have caused bias in the HbA1c measurements as well as additional variability. We could not assess the magnitude or direction of bias caused by measuring HbA1c in stored erythrocytes rather than fresh blood samples. Because this is an observational study, we cannot rule out residual or unmeasured confounding by unmeasured or poorly measured covariates. A major strength of this analysis was the high follow-up rate, limiting the potential for bias due to differential loss to follow-up.
In summary, among apparently healthy women, HbA1c measured in stored erythrocytes above 5.2% was associated with an increased risk of mortality. While some of the risk may be explained by correlates of HbA1c, including BMI, blood lipids, hypertension and hsCRP, the increased risk of mortality remained statistically significant among women with HbA1c 5.6–6.0% and no diagnosis of diabetes after controlling for these factors. These findings add further support to the hypothesis that mild hyperglycaemia, even among those without diabetes, is linked to a higher risk of mortality.
Abbreviations
- hsCRP:
-
high-sensitivity C-reactive protein
- RR:
-
relative risk
References
Garcia MJ, McNamara PM, Gordon T, Kannel WB (1974) Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes 23:105–111
Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (1997) Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20:1183–1197
Coutinho M, Gerstein HC, Wang Y, Yusuf S (1999) The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 22:233–240
Meigs JB, Nathan DM, D’Agostino RB Sr, Wilson PWF (2002) Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care 25:1845–1850
Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N (2004) Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 141:413–420
Selvin E, Coresh J, Golden SH, Brancati FL, Folsom AR, Steffes MW (2005) Glycemic control and coronary heart disease risk in persons with and without diabetes: the atherosclerosis risk in communities study. Arch Intern Med 165:1910–1916
Gerstein HC, Pogue J, Mann JF et al (2005) The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia 48:1749–1755
Levitan EB, Song Y, Ford ES, Liu S (2004) Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med 164:2147–2155
Barr EL, Zimmet PZ, Welborn TA et al (2007) Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 116:151–157
Stattin P, Bjor O, Ferrari P et al (2007) Prospective study of hyperglycemia and cancer risk. Diabetes Care 30:561–567
Saydah SH, Loria CM, Eberhardt MS, Brancati FL (2003) Abnormal glucose tolerance and the risk of cancer death in the United States. Am J Epidemiol 157:1092–1100
Saydah SH, Platz EA, Rifai N, Pollak MN, Brancati FL, Helzlsouer KJ (2003) Association of markers of insulin and glucose control with subsequent colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 12:412–418
Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N (2004) Preliminary communication: glycated hemoglobin, diabetes, and incident colorectal cancer in men and women: a prospective analysis from the European prospective investigation into cancer-Norfolk study. Cancer Epidemiol Biomarkers Prev 13:915–919
Ahmed RL, Schmitz KH, Anderson KE, Rosamond WD, Folsom AR (2006) The metabolic syndrome and risk of incident colorectal cancer. Cancer 107:28–36
Rapp K, Schroeder J, Klenk J et al (2006) Fasting blood glucose and cancer risk in a cohort of more than 140,000 adults in Austria. Diabetologia 49:945–952
International Collaborative Group (1979) Asymptomatic hyperglycemia and coronary heart disease. A series of papers by the International Collaborative Group, based on studies in fifteen populations. J Chronic Dis 32:681–837
Platz EA, Hankinson SE, Rifai N, Colditz GA, Speizer FE, Giovannucci E (1999) Glycosylated hemoglobin and risk of colorectal cancer and adenoma (United States). Cancer Causes Control 10:379–386
Blake GJ, Pradhan AD, Manson JE et al (2004) Hemoglobin A1c level and future cardiovascular events among women. Arch Intern Med 164:757–761
Lin J, Ridker PM, Pradhan A et al (2005) Hemoglobin A1c concentrations and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 14:3010–3012
Lin J, Ridker PM, Rifai N et al (2006) A prospective study of hemoglobin A1c concentrations and risk of breast cancer in women. Cancer Res 66:2869–2875
Ozasa K, Ito Y, Suzuki K et al (2005) Glucose intolerance and colorectal cancer risk in a nested case-control study among Japanese People. J Epidemiol 15(Suppl 2):S180–S184
Pradhan AD, Rifai N, Buring JE, Ridker PM (2007) Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am J Med 120:720–727
Qiao Q, Dekker JM, de Vegt F et al (2004) Two prospective studies found that elevated 2-hr glucose predicted male mortality independent of fasting glucose and HbA1c. J Clin Epidemiol 57:590–596
Nakanishi S, Yamada M, Hattori N, Suzuki G (2005) Relationship between HbA(1)c and mortality in a Japanese population. Diabetologia 48:230–234
Cook NR, Lee IM, Gaziano JM et al (2005) Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA 294:47–55
Lee IM, Cook NR, Gaziano JM et al (2005) Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA 294:56–65
Ridker PM, Cook NR, Lee IM et al (2005) A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 352:1293–1304
Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE (2000) Baseline characteristics of participants in the Women’s Health Study. J Womens Health Gend Based Med 9:19–27
Rohlfing CL, Little RR, Wiedmeyer HM et al (2000) Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care 23:187–191
Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M (2002) Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 48:436–472
American Diabetes Association (2006) Standards of Medical Care in Diabetes-2006. Diabetes Care 29:S4–S42
Rolandsson O, Marklund SL, Norberg M, Agren A, Hagg E (2004) Hemoglobin A1c can be analyzed in blood kept frozen at −80 degrees C and is not commonly affected by hemolysis in the general population. Metabolism 53:1496–1499
Selvin E, Coresh J, Jordahl J, Boland L, Steffes MW (2005) Stability of haemoglobin A1c (HbA1c) measurements from frozen whole blood samples stored for over a decade. Diabet Med 22:1726–1730
Liu S, Lee IM, Song Y et al (2006) Vitamin E and risk of type 2 diabetes in the women’s health study randomized controlled trial. Diabetes 55:2856–2862
Pickup JC (2004) Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 27:813–823
Eilers PHC, Marx BD (1996) Flexible smoothing with B-splines and penalties. Stat Sci 11:89–102
Thurston SW, Eisen EA, Schwartz J (2002) Smoothing in survival models: an application to workers exposed to metalworking fluids. Epidemiology 13:685–692
Park S, Barrett-Connor E, Wingard DL, Shan J, Edelstein S (1996) GHb is a better predictor of cardiovascular disease than fasting or postchallenge plasma glucose in women without diabetes. The Rancho Bernardo Study. Diabetes Care 19:450–456
de Vegt F, Dekker JM, Ruhe HG et al (1999) Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia 42:926–931
Calle EE, Kaaks R (2004) Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4:579–591
American Diabetes Association (2005) Diagnosis and classification of diabetes mellitus. Diabetes Care 28(Suppl 1):S37–S42
Acknowledgements
The Women’s Health Study was supported by research grants HL43851 and CA47988 from the National Institutes of Health, Bethesda, MD, USA, and by a grant from the Donald W. Reynolds Foundation, Las Vegas, NV, USA. E. B. Levitan was supported by National Heart, Lung, and Blood Institute training grant HL07374.
Duality of interest
P. M. Ridker received a research grant from Sanofi-Aventis. The other authors declare that there is no other duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Levitan, E.B., Liu, S., Stampfer, M.J. et al. HbA1c measured in stored erythrocytes and mortality rate among middle-aged and older women. Diabetologia 51, 267–275 (2008). https://doi.org/10.1007/s00125-007-0882-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-007-0882-y