Abstract
Aims/hypothesis
Low HDL-cholesterol (HDL-C) is frequently accompanied by high triacylglycerol levels in diabetic dyslipidaemia, increasing the risk of CHD. In the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study, fenofibrate treatment lowered triacylglycerol levels, but the initial 5% increase in HDL-C attenuated over 5 years. We explored the changes in VLDL and HDL subspecies during fenofibrate treatment in a statin-free FIELD cohort.
Methods
We randomised 171 participants with type 2 diabetes mellitus, who had been recruited to the FIELD study in Helsinki, to micronised fenofibrate (200 mg/day) or placebo in double-blind study design. VLDL and HDL subspecies were separated by ultracentrifugation at baseline and at the second and fifth year. Apolipoprotein (apo)A-I and apoA-II were measured by immunoturbidometric methods and lipoprotein (Lp)A-I and LpAI-AII particles by differential immunoassay.
Results
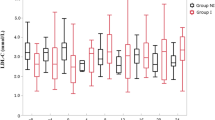
Fenofibrate reduced plasma triacylglycerol levels by 26%, resulting from a marked reduction in VLDL1 triacylglycerol (0.62 vs 0.29 mmol/l, p < 0.001). Fenofibrate caused an increase in LDL size (Δ0.80 nm, p < 0.001). HDL-C was similar between the groups. HDL2-C was decreased by fenofibrate (−27.5% at 5th year, p < 0.001) and HDL3-C increased (13.0% at 5th year, p < 0.001). Fenofibrate had no effect on apoA-I, whereas apoA-II increased. Thus, LpA-I decreased while LpAI-AII increased. Activities of cholesteryl ester transfer protein, phospholipids transfer protein and lecithin:cholesterylacyl transferase were unchanged by fenofibrate. High homocysteine levels were associated with a slight decrease in HDL-C and apoA-I.
Conclusions/interpretation
Fenofibrate markedly reduced large VLDL particles and produced a clear shift in HDL subspecies towards smaller particles. The HDL3-C increase in conjunction with unchanged apoA-II levels is a dilemma with regard to cardiovascular disease.
Similar content being viewed by others
Introduction
Clinical trials using fibrates have reported a reduction in cardiovascular events in participants with type 2 diabetes or metabolic syndrome [1–3]. The FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) study explored the effects of fenofibrate in 9,795 type 2 diabetic patients [4]. Fenofibrate treatment resulted in a 22% decrease in triacylglycerol levels, but the 11% reduction of CHD events was non-significant. Unexpectedly, a major part of the initial increase in HDL-cholesterol (HDL-C) seen at 4 months was attenuated over 5 years of fenofibrate treatment.
The HDL-C-raising effect of fenofibrate has been greater in short-term compared with long-term studies [2, 5]. HDL-C seems to increase more in non-diabetic than in diabetic participants [1, 4], but caution should be exercised when comparing studies with two different peroxisome proliferator-activated receptor α (PPARα) compounds. The vast majority of data agrees, however, that fibrates raise HDL-C primarily in the smaller and denser subclass HDL3, while the larger HDL2 remains stable or is slightly increased [6, 7]. This agrees with the proposed effects of fenofibrate on lipid-modifying enzymes. The decrease in cholesteryl ester transfer protein (CETP) should result in increased HDL-cholesterol levels, whereas increased hepatic lipase activity could partly explain the conversion of the larger HDL2 to smaller and denser HDL3 [8–10].
Low levels of large HDL particles accompanied by high levels of small HDL particles predicted higher risk of cardiovascular disease events in a recent sub-analysis of the Veterans’ Affairs HDL Intervention Trial [11]. HDL subclass distribution during fibrate treatment may thus be clinically interesting. HDL-C increases with fibrates due to increased synthesis of apolipoprotein (apo)A-I, the major apolipoprotein of HDL particles [12], and to enhanced lipolysis of triacylglycerol-rich lipoproteins (TRLs),reflected in a decreased catabolic rate of HDL particles [13].
TRL particles are readily lipolysed due to the dual action of fibrates on lipoprotein lipase (LPL) activity, namely a reduction of lipolysis-inhibiting apoCIII production and upregulation of LPL expression [13]. Whether these actions reduce the number of TRL particles or the percentage of triacylglycerol in these particles is unclear, as data on the effect of fenofibrate on separate TRL subspecies are limited [5]. TRLs consist of three major subspecies: large buoyant VLDL1, smaller and denser VLDL2, and intermediate-density lipoprotein (IDL). Of these, VLDL1-triacylglycerol strongly determine plasma triacylglycerol levels [14] and are the ancestors for the generation of small, dense LDL-C and low HDL-C levels, the two hallmarks of highly atherogenic diabetic dyslipidaemia [15].
Statin treatment is insufficient to remove the risk associated with low HDL-C levels in type 2 diabetic patients [16]. The lowering of triacylglycerol and raising of HDL-C levels are consequently gaining acceptance beyond LDL-lowering therapy. However, fenofibrate, unlike other fibrates, raises serum homocysteine levels. High homocysteine levels are associated with low apoA-I and HDL-C levels [17], probably due to a loss of PPARα-mediated transcription of apoA-I [18]. Recently, homocysteine level was reported to be a strong independent predictor of CHD events in type 2 diabetic patients [19].
The purpose of this study was to assess the effects of fenofibrate in type 2 diabetic patients on: (1) the concentration and composition of HDL subspecies 2 and 3, apoA-I, apoA-II, lipoprotein (Lp)A-I and LpAI-AII; (2) the concentration and composition of TRL subspecies VLDL1 and VLDL2, as wells as IDL and LDL size; (3) the activities of CETP, phospholipids transfer protein (PLTP) and lecithin:cholesterylacyl transferase (LCAT); and (4) to examine the effect of homocysteine on HDL.
Methods
Participants
The FIELD study design is described elsewhere [20]. Briefly, participants with type 2 diabetes mellitus aged 50 to 75 years and with serum cholesterol 3.0 to 5.5 mmol/l, plus either serum triacylglycerol 1.0–5.0 mmol/l or serum cholesterol:HDL-C ratio >4 were eligible. The patients were randomly assigned to placebo or micronised fenofibrate (200 mg per day) in a double-blind design for a period of 5 years. Statin use was allowed after randomisation. In the FIELD Helsinki Centre, 270 type 2 diabetic patients were recruited. Of these patients, 239 volunteered to participate in this substudy, of whom 228 were randomised: 113 to placebo and 115 to fenofibrate. In the second year of follow-up, 107 patients in the placebo group and 104 in the fenofibrate group were available. At substudy close at year 5 the respective numbers were 99 patients in placebo and 95 in the fenofibrate group. The reasons for drop-out during the study were 2 deaths and 12 serious adverse events in the placebo group and 5 deaths and 15 serious adverse events in the fenofibrate group. After the exclusion of additional statin users (15 in placebo vs 8 in the fenofibrate group), 84 patients in the placebo and 87 patients in the fenofibrate group were finally included in this substudy. All patients signed informed consent forms. The Ethics Committee of the Helsinki University Central Hospital approved the protocol.
Laboratory analyses
The baseline examinations were performed before fenofibrate intervention. All lipid measurements were performed in the research laboratory of the Helsinki University Central Hospital, Division of Cardiology, Helsinki, Finland. Blood samples were obtained after an overnight fast. Serum and EDTA plasma were separated by centrifugation (1,700 g) and stored at −80° until analysed. Fasting serum Lps, including HDL2 and HDL3 subspecies, were isolated from fresh serum by sequential ultracentrifugation (132,000 g) [21]. Density gradient ultracentrifugation was used to isolate TRL subspecies (192,000 g for VLDL1, 41,000 g for VLDL2, and 192,000 g for IDL) [22]. Enzymatic colorimetric assays were used to measure: cholesterol (Unimate 7 CHOL; Hoffman-La Roche, Basel, Switzerland, for baseline samples; later ABX Diagnostics Cholesterol and ABX Pentra Cholesterol, HORIBA ABX, Montpellier, France); triacylglycerol (Unimate 7 TRIG; Hoffman-La Roche for baseline samples; later ABX Diagnostics Triglycerides and ABX Pentra Triglycerides, HORIBA ABX); non-esterified cholesterol (Boehringer Mannheim, Mannheim, Germany); and phospholipid (Wako Chemicals, Neuss, Germany). Concentrations of the cholesterol were measured in whole serum and in Lp fractions using an automatic analyser (Cobas Mira; Hoffman-La Roche). Protein concentrations in the Lp subclasses were measured by a modification of the method of Lowry [23] (DC protein assay; BioRad, Hercules, CA, USA). Particle mass concentrations of the Lp subspecies were calculated by the sum of concentrations (in g/l) of triacylglycerol, non-esterified cholesterol, cholesteryl ester, phospholipids and protein. Immunoturbidimetric methods were used to measure apoB (Orion Diagnostica, Espoo, Finland), apoAI (Wako Chemicals) and apoAII (own polyclonal antibody produced in rabbits against human apoA-II). The number of LpA-I particles was measured by differential immunoassay (Sebia, Issy-les-Moulienaux, France) [24] and the number of LpAI-AII particles was calculated by subtracting the concentration of LpA-I from total apoA-I concentration, assuming that there is one apoA-1 per particle. LDL particle diameter, i.e. particle size, was measured with non-denaturing gradient gel electrophoresis [25].
Serum CETP activity was analysed after removal of endogenous VLDL and LDL by phosphotungstate-magnesium chloride precipitation [26]. For the radiometric PLTP activity assay, phosphatidylcholine small unilamellar vesicles were prepared. Each assay contained HDL3 acceptor (250 μg as protein), [14C]phosphatidylcholine-vesicles (150 nmol as phosphatidylcholine), sample (4 or 10 μl of 1:10 diluted serum) and sample buffer (10 mmol/l Tris–HCl, pH 7.4 containing 150 mmol/l NaCl, 1 mmol/l EDTA) in a final assay volume of 400 μl. Assay tubes were incubated for 45 min at 37°C, after which the excess of vesicles and plasma-derived apoB-containing Lps were precipitated with 300 μl of 215 mmol/l MnCl2–4H2O and 500 mmol/l NaCl containing 484 U/ml heparin [27]. LCAT was analysed as described previously [28]. Plasma homocysteine was determined by a fluorescence polarisation immunoassay (Abbott Laboratories, Abbott Park, IL, USA). Plasma glucose concentration was analysed by a glucose dehydrogenase method (Precision-G Blood Glucose Testing System; Medisense, Abbott Finland, Espoo, Finland) and HbA1c by a commercially available kit (DCA 2000+ Analyzer; Bayer Diagnostics, Elkhart, IN, USA).
Statistical analysis
The statistical analysis was performed using SPSS 11.0 for Windows (SPSS, Chicago, IL, USA) and CIA 2.1.2 (http://www.som.soton.ac.uk/cia/). Results are shown as median and interquartile range (IQR) due to skewed distributions of the variables. To compare the baseline data divided into quartiles by homocysteine, we used the Jonckheere–Terpstra trend-test for several samples. During treatment, nominal variables were compared using 2 × 2 likelihood ratio test. Continuous variables during treatment were compared using the Mann–Whitney U test for non-normally distributed variables: we calculated the changes in percentage from baseline and compared these changes between the treatment groups. Treatment effect is shown as median difference (+95%CI) of the group-specific relative changes. A p value of <0.05 was considered significant in all analyses.
Results
Characteristics of participants
Mean age of these patients was 62 (SD 6.5) years and their median duration of diabetes was 6 (IQR 2–11) years at baseline. Forty-five (26%) of the patients were women, 21 of them assigned to placebo and 24 to fenofibrate treatment. The treatment of blood pressure and glucose intensified during the study, as illustrated in the Electronic Supplementary Tables 1 and 2. HbA1c and fasting glucose levels remained comparable between the groups during the study, with lower fasting glucose levels at the study close compared with baseline (p = 0.007). At baseline, before any lipid-lowering medication, patients in the fenofibrate group had higher serum and LDL-cholesterol (p = 0.020 and p = 0.005, respectively) than the placebo group (Table 1). LDL-C and serum cholesterol levels decreased significantly in the fenofibrate group, whereas HDL-C levels remained comparable. Fasting serum triacylglycerol concentrations were markedly lower and LDL size higher (0.80 nm, 95%CI 0.4–1.1) in the fenofibrate group at the study close. In addition, we measured the activities of CETP, PLTP and LCAT (Table 1), which all remained unaffected by fenofibrate. Homocysteine levels expectedly increased in the fenofibrate compared with placebo group (58.8%) (Table 1).
Effect of fenofibrate on TRL subspecies
The decrease in serum triacylglycerol during fenofibrate treatment was mainly due to a marked decrease of VLDL1-triacylglycerol, with a smaller but significant decrease of VLDL2-triacylglyerol (Table 2). The decreases in triacylglycerol were in proportion to the decreases in particle mass of VLDL1 and VLDL2. Minor increases in the percentage of triacylglycerol were, however, detected in all TRL subspecies in the fenofibrate group, accompanied by decreases in the percentage of cholesteryl ester (Electronic Supplementary Table 3).
Effect of fenofibrate on HDL subspecies, apoAI and apoA-II
Fenofibrate induced opposing changes in the HDL2 and HDL3 subspecies (Table 3). HDL2-C decreased and HDL3-C increased in the fenofibrate group relative to the placebo group. The observed changes in the cholesterol levels of HDL subspecies were similar to the changes in the particle mass of HDL2 and HDL3. During the study period, levels of apoA-I remained comparable between the groups, whereas apoA-II levels increased in the fenofibrate group. This difference was reflected as a constant shift in the apoA-I-containing Lp particles, with a marked decrease in LpA-I and an increase in LpAI-AII particles in the fenofibrate group. HDL particle composition was slightly changed in the fenofibrate group, with lower cholesteryl ester (%) in HDL2 and higher non-esterified cholesterol (%) in HDL3 (Electronic Supplementary Table 4).
Impact of homocysteine levels on HDL
Since fenofibrate treatment is associated with an increase in homocysteine, we studied whether these changes would affect the HDL particles. First we examined the data from all volunteered patients before any study medication (n = 239, 32% female). We divided the patients into quartiles according to baseline homocysteine levels (Table 4). Patients in the highest quartile of homocysteine had significantly lower HDL-C, HDL3-C, apoA-II and LpAI-AII, with a non-significant tendency towards lower HDL2-C and apoA-I. Thus, higher homocysteine was associated with lower levels of primarily small HDL. Next we studied whether the increase of homocysteine during fenofibrate treatment affects HDL. The size of our study population had diminished due to drop-outs, and we divided the fenofibrate treatment group by its median homocysteine level at study close. In the fenofibrate group, low vs high homocysteine (medians 15.1 vs 23.5 μmol/l, respectively) affected HDL-C and apoA-I in opposite ways. In patients with low homocysteine levels (n = 42), HDL-C and apoA-I levels were slightly increased by fenofibrate (Δ = 0.05 mmol/l for HDL-C and Δ = 0.34 g/l for apoA-I), whereas in patients with high homocysteine levels (n = 42) HDL-C and apoA-I levels decreased (Δ = −0.04 mmol/l for HDL-C and Δ = −0.14 g/l for apoA-I) (p = 0.028 for both the differences in ΔHDL-C and ΔapoA-I between the low and high homocysteine groups).
Discussion
The FIELD study was the first long-term fenofibrate study showing the lack of durable effect on HDL-C and apoA-I levels. Although we observed no changes in HDL-C levels, there were marked changes in the distribution of HDL subspecies. A significant increase in small, dense HDL3 was accompanied by a marked decrease in large HDL2. The serum levels of apoA-I remained unaltered by fenofibrate, in accordance with the main FIELD study. Given the potent antiatherogenic function of apoA-I, this is clearly a misbenefit for the drug. Altogether these results do not support the value of fenofibrate as an HDL-raising agent. Considering that individual fibrates are different with regard to PPARα activation, their action on HDL may also be different. Despite the lack of HDL-effect, the reduction in VLDL subspecies was highly constant throughout the 5-year study. However, our study was not designed to investigate the relationship of these changes to CHD events.
Conflicting evidence exists on the atheroprotective properties of different HDL subspecies and also the molecular mechanisms are far from resolved. Mature HDL2 and HDL3 particles are acceptors in ATP-cassette binding protein G1 and scavenger receptor B1-mediated reverse cholesterol transport, with apoA-I stimulating the efflux process [29]. ApoA-I levels are determined by its production and catabolic rate, with increased catabolism of HDL apoA-I being the main cause for lowering of HDL in type 2 diabetic participants [30]. Hypercatabolism of apoA-I is linked to triacylglycerol-enrichment of HDL particles. In type 2 diabetes, as well as in our patients (Hiukka et al., unpublished data), HDL particles were rich in triacylglycerol and depleted in cholesterol [30], especially cholesteryl esters. This core lipid imbalance leads to impaired delivery of cholesteryl esters to liver and a diminished stability of apoA-I, which is essential in the efflux process [29]. In our study, fenofibrate was unable to correct the imbalance of the cholesteryl ester:triacylglycerol ratio in HDL particles (data not shown). Triacylglycerol-enriched HDL particles are highly susceptible to the lipolytic action of hepatic lipase, which is elevated in type 2 diabetes and further raised by fenofibrate [10, 31].
We observed no changes in the serum levels of apoA-I during fenofibrate treatment, in accordance with the main FIELD study. This contrasts with the concept that fenofibrate increases Apoa1 expression, as observed in mice [12]. Interestingly, fenofibrate has been reported to increase both the production and catabolism of apoA-I [32]. The paradoxical lack of increase in apoA-I levels raises the question of which mechanisms attenuate the expected effect of fenofibrate on HDL. Two recent studies in animal models have revealed a role for homocysteine in apoA-I metabolism. Homocysteine has been reported to decrease apoA-I expression, probably due to a loss of PPARα-mediated transcription [18]. Likewise, homocysteine levels in man show negative correlations with apoA-I and HDL-C levels [18]. Our baseline results agree with these findings. Moreover, we are the first to demonstrate the homocysteine-associated reduction in HDL-C and apoA-I during fenofibrate treatment. Given the minimal changes of apoA-I, the increase of homocysteine is probably only a partial explanation for fenofibrate’s lack of durable effect on HDL-C and apoA-I. The strength of this small pilot study is the detailed characterisation of the HDL subclasses 2 and 3. Whether the increase in homocysteine is associated with the cardiovascular endpoints remains to be established in further analyses of the FIELD study.
The heterogeneity of HDL subclasses has crucial implications in terms of metabolism and atheroprotective functions of HDL. HDL particles are continuously remodelled by LCAT, PLTP, CETP and lipases in circulation [33]. The effects of these proteins on lipoprotein metabolism in vivo and on atherosclerosis are complex and still poorly understood. In the present study we observed no significant changes in PLTP, CETP or LCAT activities. Thus these plasma factors do not seem to contribute to the observed changes of HDL subfractions during fenofibrate treatment. We did not measure postheparin plasma LPL and hepatic lipase activities but would expect that an increase of hepatic lipase by fenofibrate would favour increased catabolism of HDL particles. Our results contrast with recent data reporting that fenofibrate may lower CETP activity, but increase PLTP activity [8]. However, that cohort had only 11 participants and the treatment period lasted for 5 weeks.
Fenofibrate treatment expectedly increased plasma apoA-II levels. This is consistent with the well established effect of fenofibrate on APOA2 expression [13]. It should be recognised that, in contrast to apoA-I, the major predictor of plasma apoA-II levels is apoA-II production rate [34]. Our study supports the concept that increased apoA-II production results in a shift towards preponderance of smaller LpAI-AII particles. Interestingly gemfibrozil was recently reported to increase the number of HDL particles mainly due to an increase in small HDL particle number [35]. In that study HDL particle number measured by nuclear magnetic resonance (NMR) was predictive of cardiovascular benefit. Considering the strong predictive value of apoA-I for CHD risk [36], it is questionable whether the increase in HDL3-cholesterol in the setting of no change of apoA-I by fenofibrate predicts CHD events.
Plasma triacylglycerol was decreased by fenofibrate primarily due to a reduction of VLDL1-triacylglycerol in the present study. We also observed a significant, but less marked reduction in VLDL2-triacylglycerol. Thus, fenofibrate corrects the dominant defect in diabetic dyslipidaemia. Fenofibrate markedly increases the catabolism of VLDL1 particles and also the fractional transfer rate to VLDL2, while it has no effect on the production rates of VLDL1 triacylglycerol and apoB in patients with metabolic syndrome [32]. This increase in catabolism of VLDL particles occurs via PPARα activation, mediated by an increase in LPL activity together with a decrease in apoC-III synthesis. VLDL apoC-III concentration strongly determines the catabolic rate of VLDL-triacylglycerol and apoB [37]. In the current study population, fenofibrate therapy was associated with a decrease in apoC-III in all TRL subspecies (Hiukka et al., unpublished data). The decrease in apoC-III by fenofibrate is a major factor contributing to the lowering of plasma triacylglycerol [38]. Thus fibrates continue to have a role in a subset of type 2 diabetic patients with moderate to severe hypertriacylglycerolaemia, and should be preferably used in combination with statins.
Abbreviations
- apo:
-
apolipoprotein
- CETP:
-
cholesteryl ester transfer protein
- FIELD:
-
Fenofibrate Intervention and Event Lowering in Diabetes
- HDL-C:
-
HDL-cholesterol
- IDL:
-
intermediate density lipoprotein
- IQR:
-
interquartile range
- LCAT:
-
lecithin:cholesterylacyl transferase
- Lp:
-
lipoprotein
- LPL:
-
lipoprotein lipase
- PLTP:
-
phospholipids transfer protein
- PPAR:
-
peroxisome proliferator-activated receptor
- TRL:
-
triacylglycerol-rich lipoproteins
References
Rubins HB, Robins SJ, Collins D et al (2002) Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs high-density lipoprotein intervention trial (VA-HIT). Arch Intern Med 162:2597–2604
The DAIS Investigators (2001) Effect of fenofibrate on progression of coronary artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet 357:905–910
The BIP Investigators (2000) Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation 102:21–27
Keech A, Simes RJ, Barter P et al (2005) Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366:1849–1861
Milosavljevic D, Griglio S, Le Naour G, Chapman MJ (2001) Preferential reduction of very low density lipoprotein-1 particle number by fenofibrate in type IIB hyperlipidemia: consequences for lipid accumulation in human monocyte-derived macrophages. Atherosclerosis 155:251–260
Robins SJ, Collins D, Wittes JT et al (2001) Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA 285:1585–1591
Lussier-Cacan S, Bard JM, Boulet L et al (1989) Lipoprotein composition changes induced by fenofibrate in dysbetalipoproteinemia type III. Atherosclerosis 78:167–182
Watts GF, Ji J, Chan DC et al (2006) Relationships between changes in plasma lipid transfer proteins and apolipoprotein B-100 kinetics during fenofibrate treatment in the metabolic syndrome. Clin Sci (Lond) 111:193–199
Guerin M, Bruckert E, Dolphin PJ, Turpin G, Chapman MJ (1996) Fenofibrate reduces plasma cholesteryl ester transfer from HDL to VLDL and normalizes the atherogenic, dense LDL profile in combined hyperlipidemia. Arterioscler Thromb Vasc Biol 16:763–772
Desager JP, Horsmans Y, Vandenplas C, Harvengt C (1996) Pharmacodynamic activity of lipoprotein lipase and hepatic lipase, and pharmacokinetic parameters measured in normolipidaemic subjects receiving ciprofibrate (100 or 200 mg/day) or micronised fenofibrate (200 mg/day) therapy for 23 days. Atherosclerosis 124(Suppl):S65–S73
Asztalos BF, Collins D, Cupples LA et al (2005) Value of high-density lipoprotein (HDL) subpopulations in predicting recurrent cardiovascular events in the Veterans Affairs HDL Intervention Trial. Arterioscler Thromb Vasc Biol 25:2185–2191
Berthou L, Duverger N, Emmanuel F et al (1996) Opposite regulation of human vs mouse apolipoprotein A-I by fibrates in human apolipoprotein A-I transgenic mice. J Clin Invest 97:2408–2416
Keating GM, Croom KF (2007) Fenofibrate: a review of its use in primary dyslipidaemia, the metabolic syndrome and type 2 diabetes mellitus. Drugs 67:121–153
Hiukka A, Fruchart-Najib J, Leinonen E, Hilden H, Fruchart JC, Taskinen MR (2005) Alterations of lipids and apolipoprotein CIII in very low density lipoprotein subspecies in type 2 diabetes. Diabetologia 48:1207–1215
Taskinen MR (2003) Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia 46:733–749
Colhoun HM, Betteridge DJ, Durrington PN et al (2004) Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 364:685–696
Stampfer MJ, Malinow MR, Willett WC et al (1992) A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA 268:877–881
Mikael LG, Genest J, Rozen R (2006) Elevated homocysteine reduces apolipoprotein A-I expression in hyperhomocysteinemic mice and in males with coronary artery disease. Circ Res 98:564–571
Soinio M, Marniemi J, Laakso M, Lehto S, Ronnemaa T (2004) Elevated plasma homocysteine level is an independent predictor of coronary heart disease events in patients with type 2 diabetes mellitus. Ann Intern Med 140:94–100
FIELD Study Investigators (2004) The need for a large-scale trial of fibrate therapy in diabetes: the rationale and design of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. [ISRCTN64783481]. Cardiovasc Diabetol 3:9
Taskinen MR, Kuusi T, Helve E, Nikkila EA, Yki-Jarvinen H (1988) Insulin therapy induces antiatherogenic changes of serum lipoproteins in noninsulin-dependent diabetes. Arteriosclerosis 8:168–177
Taskinen MR, Packard CJ, Shepherd J (1990) Effect of insulin therapy on metabolic fate of apolipoprotein B-containing lipoproteins in NIDDM. Diabetes 39:1017–1027
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Parra HJ, Mezdour H, Ghalim N, Bard JM, Fruchart JC (1990) Differential electroimmunoassay of human LpA-I lipoprotein particles on ready-to-use plates. Clin Chem 36:1431–1435
Vakkilainen J, Jauhiainen M, Ylitalo K et al (2002) LDL particle size in familial combined hyperlipidemia: effects of serum lipids, lipoprotein-modifying enzymes, and lipid transfer proteins. J Lipid Res 43:598–603
Groener JE, Pelton RW, Kostner GM (1986) Improved estimation of cholesteryl ester transfer/exchange activity in serum or plasma. Clin Chem 32:283–286
Jauhiainen M, Ehnholm C (2005) Determination of human plasma phospholipid transfer protein mass and activity. Methods 36:97–101
Jauhiainen M, Dolphin PJ (1986) Human plasma lecithin-cholesterol acyltransferase. An elucidation of the catalytic mechanism. J Biol Chem 261:7032–7043
Kontush A, Chapman MJ (2006) Antiatherogenic small, dense HDL¯guardian angel of the arterial wall? Nat Clin Pract Cardiovasc Med 3:144–153
Frenais R, Ouguerram K, Maugeais C et al (1997) High density lipoprotein apolipoprotein AI kinetics in NIDDM: a stable isotope study. Diabetologia 40:578–583
Laakso M, Sarlund H, Ehnholm C, Voutilainen E, Aro A, Pyorala K (1987) Relationship between postheparin plasma lipases and high-density lipoprotein cholesterol in different types of diabetes. Diabetologia 30:703–706
Watts GF, Barrett PH, Ji J et al (2003) Differential regulation of lipoprotein kinetics by atorvastatin and fenofibrate in subjects with the metabolic syndrome. Diabetes 52:803–811
Huuskonen J, Olkkonen VM, Jauhiainen M, Ehnholm C (2001) The impact of phospholipid transfer protein (PLTP) on HDL metabolism. Atherosclerosis 155:269–281
Ikewaki K, Zech LA, Kindt M, Brewer HB, Jr, Rader DJ (1995) Apolipoprotein A-II production rate is a major factor regulating the distribution of apolipoprotein A-I among HDL subclasses LpA-I and LpA-I:A-II in normolipidemic humans. Arterioscler Thromb Vasc Biol 15:306–312
Otvos JD, Collins D, Freedman DS et al (2006) Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation 113:1556–1563
Barter PJ, Rye KA (2006) The rationale for using apoA-I as a clinical marker of cardiovascular risk. J Intern Med 259:447–454
Chan DC, Watts GF, Nguyen MN, Barrett PH (2006) Apolipoproteins C-III and A-V as predictors of very-low-density lipoprotein triglyceride and apolipoprotein B-100 kinetics. Arterioscler Thromb Vasc Biol 26:590–596
Lemieux I, Salomon H, Despres JP (2003) Contribution of apo CIII reduction to the greater effect of 12-week micronised fenofibrate than atorvastatin therapy on triglyceride levels and LDL size in dyslipidemic patients. Ann Med 35:442–448
Acknowledgements
The patients are thanked for participating in this study. We thank H. Hilden, R. Marjanen, V. Naatti, H. Perttunen-Nio, T. Silvennoinen, M. Tukiainen and J. Metso for their excellent technical assistance and M. Puupponen for the secretarial help. We sincerely thank S. Sarna for the statistical advice. This work was supported by grants from the Finnish Diabetes Association (E. Leinonen and M.-R.Taskinen), the Jenny and Antti Wihuri Fund (E. Leinonen), the Helsinki University Central Hospital Research Foundation (A. Hiukka, E. Leinonen and M.-R. Tasknen), the Biomedicum Helsinki Foundation (A. Hiukka), the Aarne Koskelo Foundation (A. Hiukka) and the Sigrid Juselius Foundation (M.-R. Taskinen).
Duality of interest
M.-R. Taskinen and A. C. Keech received consulting and lecture fees and grant support from Laboratoires Fournier. Since participating in this study, E. Leinonen has been appointed by Eli Lilly.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00125-007-0845-3
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hiukka, A., Leinonen, E., Jauhiainen, M. et al. Long-term effects of fenofibrate on VLDL and HDL subspecies in participants with type 2 diabetes mellitus. Diabetologia 50, 2067–2075 (2007). https://doi.org/10.1007/s00125-007-0751-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-007-0751-8