Abstract
Background
Fibrates correct the typical lipid abnormalities of type 2 diabetes mellitus, yet no study, to date, has specifically set out to evaluate the role of fibrate therapy in preventing cardiovascular events in this setting.
Methods
Subjects with type 2 diabetes, aged 50–75 years, were screened for eligibility to participate in a long-term trial of comicronized fenofibrate 200 mg daily compared with matching placebo to assess benefits of treatment on the occurrence of coronary and other vascular events. People with total cholesterol levels 3.0–6.5 mmol/L plus either a total-to-HDLc ratio >4.0 or triglyceride level >1.0 mmol/L with no clear indication for lipid-modifying therapy were eligible.
Results
A total of 9795 people were randomized into the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial. All received dietary advice, followed by a 6-week single-blind placebo run-in, then a 6-week active run-in period before randomization. Participants are being followed up every 6 months for outcome events and safety assessments. The study is designed to yield at least 500 coronary events (primary endpoint: first nonfatal myocardial infarction or coronary death) over 5 years, to have 80% power to identify as statistically significant at 2P = 0.05 a 22% reduction in such events, using intention-to-treat methods.
Conclusions
Type 2 diabetes is the most common endocrine disorder worldwide, and its prevalence is increasing. The current evidence about use of fibrates in type 2 diabetes, from around 2000 people treated, will increase with FIELD to evidence from around 12000. FIELD will establish the role of fenofibrate treatment in reducing cardiovascular risk in people with type 2 diabetes. The main results are expected to be available in late 2005.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus is an increasingly common condition associated with a high cardiovascular risk. To date, very few trials of lipid-lowering therapy have focused on this condition, and in particular, no large trials of fibrate therapy in diabetes have been conducted. As fibrates are known to correct the typical dyslipidaemia of diabetes, their role in cardiovascular risk reduction in diabetes may be especially important. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study is a multicentre, double-blind, placebo-controlled trial evaluating the effects on coronary morbidity and mortality of long-term treatment with fenofibrate to elevate high-density lipoprotein (HDL) cholesterol levels and lower triglyceride (TG) levels in patients with type 2 diabetes and total blood cholesterol between 3 and 6.5 mmol/L (115 and 250 mg/dL) at study entry. In type 2 diabetes, rates of coronary heart disease (CHD) are 3 to 4 times higher than those of persons without diabetes at any given level of blood cholesterol, and at any given age [1, 2]. Evidence also suggests that in women with diabetes the natural protection against CHD afforded by sex may be lost [3, 4]. Further, people with type 2 diabetes have both higher in-hospital mortality after myocardial infarction (MI) and a poorer outcome in the subsequent years [5, 6], losing on average between 5 and 10 years of life expectancy. It follows that type 2 diabetes contributes significantly to the overall burden of premature CHD morbidity and mortality, far in excess of its prevalence in the community.
Diabetes and blood lipids
Blood total cholesterol levels are not substantially different between patients with type 2 diabetes and those of nondiabetic populations of similar age and sex [7]. However, evaluation of other lipoprotein fractions shows that those with diabetes more often have a below-average HDL cholesterol level and elevation of TG levels in the blood [8, 9], which together confer an independent additional risk of CHD [10, 11]. Furthermore, although low-density lipoprotein (LDL) cholesterol levels are not substantially raised, the LDL particle is often smaller and denser than in similar nondiabetic populations, which is considered to be a more atherogenic state [12]. An increased number of LDL particles, as seen in diabetes, is reflected in an elevated level of plasma apolipoprotein B, a more powerful predictor of risk for cardiovascular events than either total cholesterol or LDL cholesterol [13].
The strength of the cholesterol-CHD relationship is very similar for those with type 2 diabetes as for nondiabetics, although at a higher background rate of CHD [2]. Evidence from the Helsinki Heart Study [14], which tested long-term fibrate (gemfibrozil) use in hypercholesterolaemic men and women without prior coronary disease, showed a significant reduction in coronary events, with the reduction among the small numbers of people with diabetes not being separately significant, but appearing somewhat greater [15]. The reductions in events observed were greater than would have been expected on the basis of lowering of LDL cholesterol alone. So, whether substantially increasing low HDL cholesterol levels and reducing elevated triglyceride levels independently reduces cardiovascular events and mortality and should be a specific target for therapy remains less well agreed.
Why a large trial of fibrates?
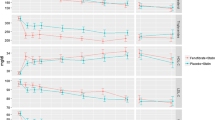
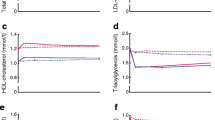
For patients with type 2 diabetes and its typical dyslipidaemia, many physicians believe that fibrates are the logical first choice of drug treatment. The fibrates have been in clinical use for a long time, being well tolerated and with few short-term side-effects. Fenofibrate has been widely used and marketed for more than 20 years and is an effective agent for reducing plasma triglyceride and raising HDL cholesterol [16]. Although the effects on lipid fractions may vary with the population under study, a fall of 15% or more in total cholesterol, mediated through a reduction in LDL cholesterol, is often seen with long-term use [16]. In parallel, HDL cholesterol elevation of 10–15% is common, together with large reductions in plasma triglycerides of 30–40%. In addition, a reduction in plasma fibrinogen of about 15% has been observed [16].
FIELD is designed to provide the first properly randomized evidence as to whether the substantial effects of fenofibrate confer a benefit on clinical cardiovascular events in persons with type 2 diabetes. A clearly favourable result might be expected to help physicians determine which type of lipid-modifying drug therapy is likely to be most cost-effective for such people.
The FIELD study design
FIELD is a randomized, double-blind, placebo-controlled parallel-group trial among middle-aged to elderly people with type 2 diabetes mellitus considered to be at increased risk of CHD. Those with and without pre-existing vascular disease or other lipid abnormalities, such as low HDL cholesterol and elevated TG, were eligible, provided the total blood cholesterol level at screening fell between 3.0 and 6.5 mmol/L (about 115–250 mg/dL) plus either a total-to-HDL cholesterol ratio of >4.0 or a blood TG level >1.0 mmol/L (88.6 mg/dL) (Table 1). The study is being conducted in 63 clinical centres in Australia (39), Finland (9) and New Zealand (15) (see Appendix).
The underlying principle guiding recruitment of patients into the study was that of clinical uncertainty: that is, patients were only to be considered if the patients' treating physicians were substantially uncertain about the value of lipid-modifying therapy for that particular individual and felt that there was no indication for lipid-modifying therapy. Therefore, none of the participants was on lipid-lowering therapy at study entry.
Following clinical and laboratory screening for eligibility, informed consent, and completion of the run-in period, patients were randomized to receive either fenofibrate (200 mg comicronized formulation) or matching placebo as one capsule daily with breakfast. There was no formal restriction on randomization related to compliance during the run-in period. Randomization was carried out using a dynamic allocation method [17] with stratification for important prognostic factors, including age, sex, prior MI, lipid levels and urinary albumin excretion. All patients are being followed up through regular clinic visits to a clinic set in place for the purposes of the study as well as by routine health care provided by a regular diabetes clinic or specialist.
The run-in phase for the study consists of a 4-week diet-only period, followed by a 6-week single-blind placebo period, then a 6-week single-blind active run-in period on comicronized fenofibrate 200 mg once daily for all patients, before randomization (Figure 1). This was to allow patients time to discuss long-term participation with their families and their usual doctors and for evaluation of the benefits of fenofibrate treatment on a background of recommended dietary advice. Further, the active run-in period was to determine to what extent any long-term clinical benefits of treatment correlate with the short-term effects of the drug to modify different lipid fractions.
Follow-up in the study will be for not less than 5 years of median duration and until a total of at least 500 first coronary events have accumulated in the trial, unless the study is terminated earlier by advice from the Safety and Data Monitoring Committee.
Study outcomes
The principal study outcome is the combined incidence of first nonfatal MI or CHD death among all randomized patients during the scheduled treatment period (Table 2). Secondary outcomes include the effects of comicronized fenofibrate on major cardiovascular events (CHD events, total stroke and other cardiovascular death combined), total cardiovascular events (major cardiovascular events plus coronary and carotid revascularization), CHD death, total cardiovascular deaths, haemorrhagic and nonhaemorrhagic stroke, coronary and peripheral revascularization procedures, cause-specific non-CHD mortality (including cancer, suicide), and total mortality. All deaths, possible MIs and possible strokes are adjudicated in blinded fashion by the Outcomes Assessment Committee.
Tertiary outcomes include the effects of treatment on development of vascular and neuropathic amputations, nonfatal cancers, the progression of renal disease, laser treatment for diabetic retinopathy, hospitalization for angina pectoris, and numbers and duration of all hospital admissions. The effects of treatment on the outcome of total cardiovascular events will be examined inThe rates of events various subgroups of particular interest, such as men and women, those <65 years and ≥ 65 years of age, by subgroup of each of baseline total cholesterol, HDL cholesterol, triglyceride and fibrinogen, baseline insulin use, or not, and the presence, or absence, at baseline of microalbuminuria.
The primary analysis will be of time to first study outcome, using standard log-rank methods [18, 19], and where appropriate, proportional-hazards models with adjustment for covariates. Intention-to-treat methods, comparing all those allocated to comicronized fenofibrate with all those allocated to placebo, will be used.
Sample size
The rates of events used for the original study power calculations were based on information from a variety of sources. During recruitment, when the numbers of participants with prior MI was falling well short of the number originally planned (in about 2000), the sample size was extended from the original total of 8000 to a final number of 9795 reached in November 2000. In late 2002, the statistical power of the trial was reviewed again. These reviews were planned in the original protocol design and were undertaken by reviewers completely blinded to all treatment allocation. The reassessment included information on final sample size, overall rate of discontinuation of study medication and commencement of open-label cholesterol treatment, and overall event rates in relation to CHD death, MI, and stroke.
After the review it was clear that the trial would yield too few deaths from CHD to retain sufficient power, over its planned duration of around 5 years, to show a significant reduction in this endpoint. The FIELD Management Committee determined that the primary outcome of the trial should be amended from CHD death to CHD events (that is, CHD death plus nonfatal MI, a decision made in December 2002). It was also decided to change the principal outcome for subgroup analyses to look at the effects of fenofibrate in subjects with and without prior cardiovascular disease (CVD) (originally those with and without prior MI).
For a primary outcome of CHD events (CHD death plus nonfatal MI), it is projected that approximately 500 CHD events will have occurred when 5 years median follow-up has elapsed (during the first quarter of 2005); by this time the trial will have 80% power to detect an observed 22% reduction in CHD events (based on the intention-to-treat method of analysis). This will also provide 90% power to detect a 25% relative reduction in CHD events (based on intention-to-treat analysis). Both calculations allow for the effects of an average drop-out rate from active treatment over the course of the study of 10% and a similar drop-in rate of 10% from placebo to open cholesterol-lowering therapy (Table 3). These allowances for loss of compliance require an increase in sample size of approximately 60% when compared with a study with no drop-outs from, or drop-ins to, active treatment.
If the uptake of cholesterol-lowering therapy proves to be greater in the placebo group than in the fenofibrate-allocated group, the observed treatment effect of fenofibrate will underestimate its true efficacy.
Safety and event monitoring
The trial has an independent Safety and Data Monitoring Committee to safeguard the patients' interests and to formally evaluate from time to time on a regular basis whether, for any reason, they would recommend that the study should be modified or stopped. Up to 5 formal interim analyses are planned, at time points to be determined by the Safety and Data Monitoring Committee, with a stringent nominal significance level (3 standard deviations; 2P = 0.003) to preserve an overall type 1 error probability of no more than 0.05. The events to be used for these analyses are counts of death from CHD. The Management Committee, the collaborators, the study sponsor and all the central administrative staff, with the exception of the unblinded statistician, will remain ignorant of the interim results for mortality and major morbidity. During the study, the group effects of treatment on biochemical parameters, such as lipid fractions, and other surrogate endpoints may be published, subject to prior approval of the Management Committee, provided that individual patient treatment assignments are not revealed. Patients are being monitored regularly by lipid profiles, liver function tests, creatine phosphokinase, fasting glucose, HbA1c, and urinary microalbumin. The study has been approved by local ethics committees at each participating institution, which also approved the information discussed and informed-consent procedures. The first patient in FIELD was registered in November 1997 and randomized in February 1998. The study has recruited 9795 patients; the final patient was randomized on 3 November 2000.
Study sponsorship and organisation
The main sponsor of the trial and supplier of the fenofibrate and matching placebo medication, is Laboratoires Fournier S.A., Dijon, France. The study is also supported by the National Health and Medical Research Council of Australia through Unit, Program and Fellowship grants to the NHMRC Clinical Trials Centre. The study is being coordinated independently of the sponsors by the NHMRC Clinical Trials Centre, University of Sydney, Sydney, Australia and overseen by the study Management Committee. The study has been endorsed by the National Heart Foundation of Australia, Diabetes Australia, the New Zealand Society for the Study of Diabetes, and the Finnish Diabetes Association.
Conclusion
In 1997, before the FIELD study commenced, the role of lipid modification in diabetes remained uncertain, except possibly for hypercholesterolaemic people with a prior MI. Two large-scale trials, the Scandinavian Simvastatin Survival Study (4S) [20] and the Cholesterol and Recurrent Events (CARE) [21] study, had showed that the use of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, simvastatin and pravastatin, respectively, substantially reduced cardiovascular events hypercholesterolaemic and in general post-MI populations. But neither study included sufficient numbers of patients with diabetes (n = 202 and n = 586, respectively) to have the power to show reliably whether these benefits would translate into reductions in CHD mortality in the setting of diabetes, nor the effects in them of treatment on noncoronary events and mortality. Further, the West of Scotland (WOSCOPS) study of pravastatin in hypercholesterolaemic men with no prior CHD, which reported a marginally significant reduction in overall mortality, had fewer than 100 subjects with diabetes [22].
Since that time, numerous other trials of statin treatment have been reported, with randomized data now reported on over 18000 persons with diabetes. Those involving more than 1000 people with diabetes include the Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) study [6, 23], the Heart Protection Study [24, 25], the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) [26] and Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) [27]. Another trial, the Collaborative Atorvastatin Diabetes Study (CARDS), has stopped early, after about 4 years of follow-up, with results showing clear benefits of reduced cardiac and stroke events of using atorvastatin among 2838 people with diabetes and high cardiovascular risk [28]. Important new results have been communicated to investigators and patients so that it can be considered whether, during the follow-up of FIELD, statin therapy is now indicated for any individual. The protocol allows for statin therapy to be added at any time after randomization and recommends continuing study medication; thus the study is evaluating the role of fenofibrate on a background of usual care. This feature of the study design will contribute to the evidence about the safety of combined statin and fibrate therapy.
Two large-scale trials of fibrate therapy have also been completed: the Veterans Low-HDL Cholesterol Intervention Trial (VA-HIT) [29, 30] and the Bezafibrate Infarct Prevention (BIP) [31] trial. Both studies were limited to people with prior MI and have reported reductions in major cardiovascular events among participants with low HDL and high TG at baseline, which were greater than those seen with use of the same fibrate among those without dyslipidaemia. The VA-HIT trial also reported reduced CHD mortality in those with diabetes receiving gemfibrozil and a reduced rate of cardiovascular events, although rates of nonfatal MI did not change significantly [32]. A third trial, the Diabetes Atherosclerosis Intervention Study (DAIS), showed reduced progression of established coronary atherosclerosis among those randomized to fenofibrate compared with those receiving matching placebo, over 3 years [33].
At the same time, our understanding of the mechanism of action of fibrates has grown, with identification of the peroxisome proliferator-activated receptor alpha (PPAR-alpha) transcription factor as the primary pathway through which fibrate-mediated effects are triggered [34, 35]. The abundance of desirable effects of PPAR-alpha activation by fibrates has generated extraordinary interest in their role in the prevention of atherosclerosis via regulation of lipid metabolism, vascular inflammation, and haemostatic factors. The importance of changes in apolipoprotein B and non-HDL-cholesterol levels appears greater with fibrate therapy than with statin use [36], particularly in patients with type 2 diabetes [37]. Increased interest in the FIELD study has resulted, as it will generate clinical data on similar numbers of persons with diabetes to that available for the statins (Table 4) and will enlarge the range of lipid profiles studied and the number of events in such populations (Table 5).
Approximately 140 million adults were estimated to be suffering from diabetes mellitus, the most common endocrine disorder worldwide, in 1997. By 2010, projections put diabetes prevalence about 60 percent higher, at 221 million. Just as many persons again have an elevated fasting glucose level, or impaired fasting glucose, which can progress rapidly to diabetes. Without the FIELD study, doctors would remain uncertain about the merits of using a fibrate when confronted with a patient with diabetes at risk of clinical CHD. It is expected that the main results of FIELD will be reported in late 2005.
Appendix 1: Study organization
Management Committee
P Barter*, J Best*, P Colman, M d’Emden, T Davis, P Drury, C Ehnholm, P Glasziou, D Hunt, A Keech* (study chairman and principal investigator), YA Kesaniemi, M Laakso, R Scott*, RJ Simes*, D Sullivan, M-R Taskinen*, M Whiting; J-C Ansquer, B Fraitag (non-voting sponsor representatives). * Executive Committee members.
Outcomes Assessment Committee
N Anderson, G Hankey, D Hunt (chairman), S Lehto, S Mann, M Romo; LP Li (outcomes officer, in attendance).
Safety and Data Monitoring Committee
C Hennekens, S MacMahon (chairman), S Pocock, A Tonkin, L Wilhelmsen; P Forder (unblinded statistician, in attendance).
Site principal investigators
Australia: H Akauola, F Alford, P Barter, I Beinart, J Best, S Bohra, S Boyages, P Colman, H Connor, D Darnell, T Davis, P Davoren, F Lepre, F De Looze, M d'Emden, A Duffield, R Fassett, J Flack, G Fulcher, S Grant, S Hamwood, D Harmelin, R Jackson, W Jeffries, M Kamp, L Kritharides, L Mahar, V McCann, D McIntyre, R Moses, H Newnham, G Nicholson, R O'Brien, K Park, N Petrovsky, P Phillips, G Pinn, D Simmons, K Stanton, B Stuckey, D R Sullivan, M Suranyi, M Suthers, Y Tan, M Templer, D Topliss, J H Waites, G Watts, T Welborn, R Wyndham; Finland: H Haapamaki, A Kesaniemi, M Laakso, J Lahtela, H Levanen, J Saltevo, H Sodervik, M Taskinen, M Vanhala; New Zealand: J Baker, A Burton, P Dixon, J Doran, P Drury, P Dunn, N Graham, A Hamer, J Hedley, J Lloyd, P Manning, I McPherson, S Morris, C Renner, R Scott, R Smith, M Wackrow, S Young.
Co-investigators and site coordinators
Australia: F Alard, J Alcoe, F Alford, C Allan, J Amerena, R Anderson, N Arnold, T Arsov, D Ashby, C Atkinson, L Badhni, M Balme, D Barton, B Batrouney, C Beare, T Beattie, J Beggs, C Bendall, C Bendall, A Benz, A Bond, R Bradfield, J Bradshaw, S Brearley, D Bruce, J Burgess, J Butler, M Callary, J Campbell, K Chambers, J Chow, S Chow, K Ciszek, P Clifton, P Clifton-Bligh, V Clowes, P Coates, C Cocks, S Cole, D Colquhoun, M Correcha, B Costa, S Coverdale, M Croft, J Crowe, S Dal Sasso, W Davis, J Dunn, S Edwards, R Elder, S El-Kaissi, L Emery, M England, O Farouque, M Fernandez, B Fitzpatrick, N Francis, P Freeman, A Fuller, D Gale, V Gaylard, C Gillzan, C Glatthaar, J Goddard, V Grange, T Greenaway, J Griffin, A Grogan, S Guha, J Gustafson, P S Hamblin, T Hannay, C Hardie, A Harper, G Hartl, A Harvey, S Havlin, K Haworth, P Hay, L Hay, B Heenan, R Hesketh, A Heyworth, M Hines, G Hockings, A Hodge, L Hoffman, L Hoskin, M Howells, D Hunt, A Hunt, W Inder, W Inder, D Jackson, A Jovanovska, K Kearins, P Kee, J Keen, D Kilpatrick, J Kindellan, M Kingston-Ray, M Kotowicz, A Lassig, M Layton, S Lean, E Lim, F Long, L Lucas, D Ludeman, D Ludeman, C Ludeman-Robertson, M Lyall, L Lynch, C Maddison, B Malkus, A Marangou, F Margrie, K Matthiesson, J Matthiesson, S Maxwell, K McCarthy, A McElduff, H McKee, J McKenzie, K McLachan, P McNair, M Meischke, A Merkel, C Miller, B Morrison, A Morton, W Mossman, A Mowat, J Muecke, P Murie, S Murray, P Nadorp, S Nair, J Nairn, A Nankervis, K Narayan, N Nattrass, J Ngui, S Nicholls, V Nicholls, JA Nye, E Nye, D O'Neal, M O'Neill, S O'Rourke, J Pearse, C Pearson, J Phillips, L Pittis, D Playford, L Porter, L Porter, R Portley, M Powell, C Preston, S Pringle, W A Quinn, J Raffaele, G Ramnath, J Ramsden, D Richtsteiger, S Roffe, S Rosen, G Ross, Z Ross, J Rowe, D Rumble, S Ryan, J Sansom, C Seymour, E Shanahan, S Shelly, J Shepherd, G Sherman, R Siddall, D Silva, S Simmons, R Simpson, A Sinha, R Slobodniuk, M Smith, P Smith, S Smith, V Smith-Orr, J Snow, L Socha, T Stack, K Steed, K Steele, J Stephensen, P Stevens, G Stewart, R Stewart, C Strakosch, M Sullivan, S Sunder, J Sunderland, E Tapp, J Taylor, D Thorn, D Thorn, A Tolley, D Torpy, G Truran, F Turner, J Turner, J van de Velde, S Varley, J Wallace, J Walsh, J Walsh, J Walshe, G Ward, B Watson, J Watson, A Webb, F Werner, E White, A Whitehouse, N Whitehouse, S Wigg, J Wilkinson, E Wilmshurst, D Wilson, G Wittert, B Wong, M Wong, S Worboys, S Wright, S Wu, J Yarker, M Yeo, K Young, J Youssef, R Yuen, H Zeimer, R W Ziffer; Finland: A Aura, A Friman, J Hanninen, J Henell, N Hyvarinen, M Ikonen, A Itkonen, J Jappinen, A Jarva, T Jerkkola, V Jokinen, J Juutilainen, H Kahkonen, T Kangas, M Karttunen, P Kauranen, S Kortelainen, H Koukkunen, L Kumpulainen, T Laitinen, M Laitinen, S Lehto, R Lehto, E Leinonen, M Lindstron-Karjalainen, A Lumiaho, J Makela, K Makinen, L Mannermaa, T Mard, J Miettinen, V Naatti, S Paavola, N Parssinen, J Ripatti, S Ruotsalainen, A Salo, M Siiskonen, A Soppela, J Starck, I Suonranta, L Ukkola, K Valli, J Virolainen; New Zealand: P Allan, W Arnold, W Bagg, K Balfour, T Ball, B Ballantine, C Ballantyne, C Barker, C Barker, F Bartley, E Berry, G Braatvedt, A Campbell, T Clarke, R Clarke, A Claydon, S Clayton, P Cresswell, R Cutfield, J Daffurn, J Delahunt, A Dissnayake, C Eagleton, C Ferguson, C Florkowski, D Fry, P Giles, M Gluyas, C Grant, P Guile, M Guolo, P Hale, M Hammond, M Hammond, P Healy, M Hills, J Hinge, J Holland, B Hyne, A Ireland, A Johnstone, S Jones, G Kerr, K Kerr, M Khant, J Krebs, L Law, B Lydon, K MacAuley, R McEwan, P McGregor, B McLaren, L McLeod, J Medforth, R Miskimmin, J Moffat, M Pickup, C Prentice, M Rahman, E Reda, C Ross, A Ryalls, D Schmid, N Shergill, A Snaddon, H Snell, L Stevens, A Waterman, V Watts.
Coordinating centre teams
NHMRC Clinical Trials Centre, Sydney: K Jayne, E Keirnan, P Newman, G Ritchie, A Rosenfeld (project directors), E Beller, P Forder, V Gebski, A Pillai (study statisticians), C Anderson, S Blakesmith, S-Y Chan, S Czyniewski, A Dobbie, S Doshi, A Dupuy, S Eckermann, M Edwards, N Fields, K Flood, S Ford, C French, S Gillies, C Greig, M Groshens, J Gu, Y Guo, W Hague, S Healy, L Hones, Z Hossain, M Howlett, J Lee, L-P Li, T Matthews, J Micallef, A Martin, I Minns, A Nguyen, F Papuni, A Patel, J Pearse, R Pike, M Pena, K Pinto, D Schipp, J Schroeder, B Sim, C Sodhi, T Sourjina, C Sutton, R Taylor, P Vlagsma, S Walder, R Walker, W Wong, J Zhang, B Zhong, A Keech (deputy director), RJ Simes (director); Helsinki Project Office: A Kokkonen, P Narva, E-L Niemi, A Salo, A-M Syrjanen, M-R Taskinen (director); Christchurch Project Office: C Lintott, R Scott (director).
Central laboratories
Adelaide: R Tirimacco, M Whiting; Helsinki: C Ehnholm, M Ikonen, M Kajosaari, L Raman, J Sundvall, M Tukianen. .
Laboratoires Fournier SA liaison: Dijon: J-C Ansquer, B Fraitag, D Crimet, I SirugueSydney: P Aubonnet.
References
Barrett-Connor E, Orchard T: Diabetes and heart disease. In: Diabetes Data Compiled 1984. National Diabetes Data Group. NIH publication no. 85-1468. Washington DC: US Department of Health and Human Services, XVI-XVI-41. 1985
Stamler J, Vaccaro O, Neaton JD, Westworth D: Diabetes and other risk factors and the 12-year cardiovascular mortality for men screened for the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993, 16: 434-444.
Kannel WB: Lipids, diabetes and coronary disease from the Framingham Study. Am Heart J. 1985, 110: 1100-1107. 10.1016/0002-8703(85)90224-8.
Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL: Why is diabetes mellitus a stronger risk factor for fatal ischaemic heart disease in women than in men? The Rancho Bernardo Study. JAMA. 1991, 265: 627-631. 10.1001/jama.265.5.627.
Zuanetti G, Latini R, Maggioni AP, Santoro L, Franzosi MG: Influence of diabetes on mortality in acute myocardial infarction: data from the GISSI-2 study. J Am Coll Cardiol. 1993, 22: 1788-1794.
Keech AC, Colquhoun D, Best J, Kirby A, Simes RJ, Hunt D, Hague W, Beller E, Arulchelvam M, Baker J, Tonkin A, for the LIPID study group: Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose: results from the LIPID trial. Diabetes Care. 2003, 26: 2713-2721.
Laakso M, Voutilainen E, Sarlund H, Aro A, Pyorala K, Penttila I: Serum lipids and lipoproteins in middle aged non-insulin dependent diabetics. Atherosclerosis. 1985, 56: 271-281.
Wilson PWF, Kannel WB, Anderson KM: Lipids, glucose intolerance and vascular disease: The Framingham Study. Monogr Atheroscler. 1985, 13: 1-11.
Howard BV: Lipoprotein metabolism in diabetes mellitus. J Lipid Res. 1987, 28: 613-628.
Fontbonne A, Eschwege E, Cambien F, Richard JL, Ducimetiere P, Thibult N, Warnet JM, Claude JR, Rosselin GE: Hypertriglyceridemia as a risk factor for coronary heart disease mortality in subjects with impaired glucose tolerance or diabetes. Diabetologia. 1989, 32: 300-304. 10.1007/BF00265546.
Assmann G, Schulte H: Triglycerides and atherosclerosis; results from the Prospective Cardiovascular Munster Study. Arterioscler Rev. 1991, 22: 51-57.
Feingold KR, Grunfeld C, Pang M, Doerrler W, Krauss RM: LDL subclass phenotypes and triglyceride metabolism in non-insulin dependent diabetes. Arterioscler Thromb. 1992, 12: 1496-1502.
Sniderman AD, Furberg CD, Keech A, Roeters van Lennep JE, Frohlich J, Jungner I, Walldius G: Apolipoproteins versus lipids as indices of coronary risk and as targets for statin treatment. Lancet. 2003, 361: 777-780. 10.1016/S0140-6736(03)12663-3.
Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V, and others: Helsinki Heart Study: primary prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987, 317: 1237-1245.
Koskinen P, Mänttäri M, Manninen V, Huttunen JK, Heinonen OP, Frick MH: Coronary heart disease incidence in NIDDM patients in the Helsinki Heart Study. Diabetes Care. 1992, 15: 820-825.
Keating GM, Ormrod D: Micronised fenofibrate. An updated review of its clinical efficacy in the management of dyslipidaemia. Drugs. 2002, 62: 1909-1944.
Signorini DF, Leung O, Simes RJ, Beller E, Gebski VJ, Callaghan T: Dynamic balanced randomizatio for clinical trials. Stat Med. 1993, 12: 2343-2250.
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG: Design and analysis of randomised clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976, 34: 585-612.
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG: Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer. 1977, 35: 1-39.
Scandinavian Simvastatin Survival Study Group: Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease; the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994, 344: 1383-1389.
Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E: The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996, 335: 1001-1009. 10.1056/NEJM199610033351401.
Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ: Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995, 333: 1301-1307. 10.1056/NEJM199511163332001.
LIPID Study Group: Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998, 339: 1349-1357. 10.1056/NEJM199811053391902.
Heart Protection Study Collaborative Group: MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002, 360: 7-22. 10.1016/S0140-6736(02)09327-3.
Heart Protection Study Collaborative Group: MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003, 361: 2005-2016. 10.1016/S0140-6736(03)13636-7.
Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J, ASCOT investigators: Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003, 361: 1149-1158. 10.1016/S0140-6736(03)12948-0.
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial:: Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002, 288: 2998-3007. 10.1001/jama.288.23.2998.
Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HAW, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH, on behalf of the CARDS Investigators: Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004, 364: 685-696. 10.1016/S0140-6736(04)16895-5.
Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J: Gemfibrozil for secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-density lipoprotein cholesterol Intervention Trial study group. N Engl J Med. 1999, 341: 410-418. 10.1056/NEJM199908053410604.
Robins SJ, Collins D, Wittes JT, Papademetriou V, Deedwania PC, Schaefer EJ, McNamara JR, Kashyap ML, Hershman JM, Wexler LF, Rubins HB: Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA. 2001, 285: 1585-1591. 10.1001/jama.285.12.1585.
The BIP Study Group: Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. The Bezafibrate Infarction Prevention (BIP) Study. Circulation. 2000, 102: 21-27.
Rubins HB, Robins SJ, Collins D, Nelson DB, Elam MB, Schaefer EJ, Faas FH, Anderson JW, for the VA-HIT Study Group: Diabetes, plasma insulin and cardiovascular disease. Subgroup analysis from the Department of Veterans Affairs high-density lipoprotein intervention trial (VA-HIT). Arch Intern Med. 2002, 162: 2597-2604. 10.1001/archinte.162.22.2597.
Diabetes Atherosclerosis Intervention Study Investigators: Effect of fenofibrate on progression of coronary artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet. 2001, 357: 905-910. 10.1016/S0140-6736(00)04209-4.
Schoonjans K, Staels B, Auwerx J: Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res. 1996, 37: 907-925.
Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC: Mechanisms of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998, 98: 2088-2093.
Aguilar-Salinas CA, Fanghanel-Salmon G, Meza E, Montes J, Gulias-Herrero A, Sanchez L, Monterrubio-Flores EA, Gonzalez-Valdez H, Gomez Perez FJ: Ciprofibrate versus gemfibrozil in the treatment of mixed hyperlipidemias: an open-label, multicenter study. Metabolism. 2001, 50: 729-733. 10.1053/meta.2001.23308.
Branchi A, Rovellini A, Torri A, Sommariva D: Accuracy of calculated serum low-density lipoprotein cholesterol for the assessment of coronary heart disease risk in NIDDM patients. Diabetes Care. 1998, 21: 1397-1402.
Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G, for the Scandinavian Simvastatin Survival Study (4S) Group: Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease: A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997, 20: 614-620.
Post Coronary Artery Bypass Graft Trial Investigators: The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. N Engl J Med. 1997, 336: 153-62. 10.1056/NEJM199701163360301.
Hoogwerf BJ, Waness A, Cressman M, Canner J, Campeau , Domanski M, Geller N, Herd A, Hickey A, Hunninghake DB, Knatterud GL, White C: Effects of aggressive cholesterol lowering and low-dose anticoagulation on clinical and angiographic outcomes in patients with diabetes. The Post Coronary Artery Bypass Graft Trial. Diabetes. 1999, 48: 1289-1294.
GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico): Results of the low-dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge?. Ital Heart J. 2000, 1: 810-20.
Athyros VG, Mikhailidis DP, Papageorgiou AA, Mercouris BR, Athyrou VV, Symeonidis AN, Basayannis EO, Demitriadis DS, Kontopoulos AG: Attaining United Kingdom-European Atherosclerosis Society low-density lipoprotein cholesterol guideline target values in the Greek Atorvastatin and Coronary-Heart-Disease Evaluation (GREACE) Study. Curr Med Res Opin. 2002, 18: 499-502. 10.1185/030079902125001317.
Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG, PROSPER study group: Prospective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002, 360: 1623-630. 10.1016/S0140-6736(02)11600-X.
Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA Langendorfer A, Stein EA, Kruyer W, Gotto AM: Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. Results of AFCAPS/TexCAPS. JAMA. 1998, 279: 1615-1622. 10.1001/jama.279.20.1615.
Meade T, Zuhrie R, Cook C, Cooper J, on behalf of MRC General Practice Research Framework: Bezafibrate in men with lower extremity arterial disease: randomized controlled trial. BMJ. 2002, 325: 1139-10.1136/bmj.325.7373.1139.
Elkeles RS, Diamond JR, Poulter C, Dhanjil S, Nicolaides A, Mahmood S, Richmond W, Mather H, Sharp P, Feher MD: Cardiovascular outcomes in type 2 diabetes: A double-blind placebo-controlled study of bezafibrate: The St. Mary's, Ealing, Northwick Park Diabetes Cardiovascular Disease Prevention (SENDCAP) Study. Diabetes Care. 1998, 21: 641-642.
Acknowledgements
This study is supported by a grant from Laboratoires Fournier SA, Dijon, France and is being coordinated independently by the National Health and Medical Research Council Clinical Trials Centre, University of Sydney, Sydney, Australia, and overseen by the study Management Committee.
The study is also supported by the National Health and Medical Research Council (NHMRC), Australia (Unit grant, Project grant and Fellowships to A. Keech and J. Simes), without which it would not be possible. We thank the National Heart Foundation, Australia, Diabetes Australia, Diabetes New Zealand, and the Finnish Diabetes Association for endorsing the study. Investigators express their thanks to Rhana Pike and Christelle Foucher for their assistance with the preparation of this manuscript, and the staff of Kadima, Sydney, for efficient management and distribution of study materials. Finally, the many patients participating in the FIELD study are thanked for their untiring contributions.
Author information
Authors and Affiliations
Consortia
Additional information
Declaration of competing interests
Of the Management Committee of the FIELD Study:
PB, YAK and RS have received reimbursements, fees, funding, or salary in the past five years from an organization that may in any way gain or lose financially from the publication of this paper;
No authors hold or have held stocks or shares in such an organization;
No authors have other financial competing interests;
AK has the following nonfinancial competing interests: Advisory board membership.
Authors' contributions
The FIELD Management Committee conceived and developed the study protocol and are the responsible authors of this manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
The FIELD Study Investigators. The need for a large-scale trial of fibrate therapy in diabetes: the rationale and design of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. [ISRCTN64783481]. Cardiovasc Diabetol 3, 9 (2004). https://doi.org/10.1186/1475-2840-3-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2840-3-9