Abstract
Aims/hypothesis
We evaluated the association of QT interval corrected for heart rate (QTc) and resting heart rate (rHR) with mortality (all-causes, cardiovascular, cardiac, and ischaemic heart disease) in subjects with type 1 and type 2 diabetes.
Methods
We followed 523 diabetic patients (221 with type 1 diabetes, 302 with type 2 diabetes) who were recruited between 1974 and 1977 in Switzerland for the WHO Multinational Study of Vascular Disease in Diabetes. Duration of follow-up was 22.6 ± 0.6 years. Causes of death were obtained from death certificates, hospital records, post-mortem reports, and additional information given by treating physicians.
Results
In subjects with type 1 diabetes QTc, but not rHR, was associated with an increased risk of: (1) all-cause mortality (hazard ratio [HR] 1.10 per 10 ms increase in QTc, 95% CI 1.02–1.20, p = 0.011); (2) mortality due to cardiovascular (HR 1.15, 1.02–1.31, p = 0.024); and (3) mortality due to cardiac disease (HR 1.19, 1.03–1.36, p = 0.016). Findings for subjects with type 2 diabetes were different: rHR, but not QTc was associated with mortality due to: (1) all causes (HR 1.31 per 10 beats per min, 95% CI 1.15–1.50, p < 0.001); (2) cardiovascular disease (HR 1.43, 1.18–1.73, p < 0.001); (3) cardiac disease (HR 1.45, 1.19–1.76, p < 0.001); and (4) ischaemic heart disease (HR 1.52, 1.21–1.90, p < 0.001). Effect modification of QTc by type 1 and rHR by type 2 diabetes was statistically significant (p < 0.05 for all terms of interaction).
Conclusions/interpretation
QTc is associated with long-term mortality in subjects with type 1 diabetes, whereas rHR is related to increased mortality risk in subjects with type 2 diabetes.
Similar content being viewed by others
Introduction
Compared with the non-diabetic population, subjects with type 1 and type 2 diabetes mellitus are reported to have an increase in all-cause mortality [1]. Cardiovascular disease has been found to be the main reason for this excess mortality [1–4]. In an effort to identify easily available and reliable predictors for cardiovascular risk and mortality in diabetes mellitus, the evaluation of parameters reflecting myocardial ventricular repolarisation has been of particular interest. In subjects with type 1 diabetes, prolongation of the QT interval corrected for heart rate (QTc) and heart rate variability have both been shown to be associated with increased risk of arrhythmia and death, whereas QT dispersion has been suggested to be less reliable [5]. However, the association between QTc and cardiovascular mortality in type 2 diabetes is controversial. Some reports suggest that QTc correlates with an increase in cardiovascular mortality [6–9]. Others have indicated that QT dispersion might more accurately predict cardiovascular mortality in this patient group [10–12].
Increased resting heart rate (rHR), which is easily measurable in clinical practice, has been shown to be an independent risk factor for cardiovascular death in a non-diabetic population [13–16]. Recently, rHR has also been shown to be valuable in estimating the risk of cardiovascular death in patients with type 2 diabetes [8, 10]. However, data directly comparing the role of QTc and rHR in subjects with type 1 and type 2 diabetes are lacking. Based on a 23-year follow-up of the Swiss cohort of the WHO Multinational Study of Vascular Disease in Diabetes [17], the present study aimed to evaluate the long-term association of QTc and rHR with mortality in patients with type 1 and type 2 diabetes within the same study framework.
Subjects and methods
Study population
The WHO Multinational Study of Vascular Disease in Diabetes is a multicentre international study with a central protocol applied by 14 centres in 13 countries [18]. For the original study, each centre recruited stratified samples of 250 men and 250 women with a clinical diagnosis of diabetes, aged between 35 and 54 years at time of recruitment. The present analysis was based on the Swiss cohort of this study [17], which included 533 subjects randomly selected according to the central protocol by 231 local practitioners [17, 18]. The sample was representative of a large area including almost the entire country. The diagnosis of diabetes mellitus was made on a clinical basis. Subjects were eligible if diabetes had been diagnosed at least 1 year prior to study entry and anti-diabetic treatment (diet, oral glucose-lowering drugs, insulin) had been initiated by their physicians. If insulin was needed for treatment within 1 year of diagnosis, subjects were considered to have type 1 diabetes [18], the remaining subjects were classified as having type 2 diabetes. These comparably simple clinical definitions with acknowledged inadequacies were used because of the constraints on information available and the need for consistency with earlier reports [19–21]. At baseline, a standardised clinical examination was performed, including a detailed questionnaire with information on diabetes diagnosis, the duration and treatment, as well as on symptoms of vascular and cardiac disease. Previous medical history also included the use of other medication (including diuretics, lipid-lowering drugs and blood pressure-lowering drugs). Central randomisation to the cohort was stratified according to sex, age (35–41 years, 42–48 years, 49–54 years) and duration of diabetes (1–6 years, 7–13 years, 14 years and more). In addition, height and weight were recorded, and blood pressure was measured after 30 min of rest; hypertension being defined as a systolic blood pressure of ≥160 mmHg, and/or a diastolic blood pressure ≥95 mmHg, and/or the use of antihypertensive medication including diuretics. Urine was tested semiquantitatively for proteinuria using the salicylsulfonic acid method, blood samples were drawn to measure fasting plasma glucose, cholesterol, triacylglycerol and creatinine, and a 12-lead ECG was recorded. These baseline investigations were carried out between February 1974 and May 1977. All subjects gave informed consent. Analyses were carried out in accordance with the Declaration of Helsinki and the Swiss laws regarding data security. Data used were made fully anonymous before the analyses.
ECG recordings
Standard 12-lead resting ECGs were recorded with the patient supine and resting for at least 30 min. Analyses were performed according to the Minnesota code [22]. All tracings were evaluated by the same two experienced readers. The following items were derived from the ECG code results: ‘ECG coronary probable’ consisting of code 1.1, 1.2 and 7.1; ‘ECG coronary possible’ consisting of codes 1.3, 4.1, 4.2, 4.3, 5.1, 5.2 and 5.3; all other recordings were rated as ‘ECG coronary unlikely’ [22]. QT and RR intervals were measured by an experienced cardiologist, blinded to the diagnosis and outcome of the individual patients. QT interval length was usually measured in the ventral (V)2 and V3 leads using a digitiser (CalComp, Newbury, Berks, UK). Measurements in V2 and V3 were chosen, since they provide a close approximation of maximal QT [23]. QT interval length was measured from the onset of the QRS to the end of the T wave. In the presence of U waves, the end of the QT interval was set at the nadir of the curve between T and U wave. Maximal QT interval was corrected for the respective heart rate using the Bazett formula [24] \( {\left( {{\text{QT}}_{{{\text{c}}\,{\text{Bazett}}}} = {{\text{QT}}} \mathord{\left/ {\vphantom {{{\text{QT}}} {{\text{RR}}^{{{\text{1}} \mathord{\left/ {\vphantom {{\text{1}} {\text{2}}}} \right. \kern-\nulldelimiterspace} {\text{2}}}} }}} \right. \kern-\nulldelimiterspace} {{\text{RR}}^{{{\text{1}} \mathord{\left/ {\vphantom {{\text{1}} {\text{2}}}} \right. \kern-\nulldelimiterspace} {\text{2}}}} }} \right)} \). In addition, the formulas suggested by Fridericia \( {\left( {{\text{QT}}_{{{\text{c}}\,{\text{Fridericia}}}} {\text{ = }}{{\text{QT}}} \mathord{\left/ {\vphantom {{{\text{QT}}} {{\text{RR}}^{{{\text{1}} \mathord{\left/ {\vphantom {{\text{1}} {\text{3}}}} \right. \kern-\nulldelimiterspace} {\text{3}}}} }}} \right. \kern-\nulldelimiterspace} {{\text{RR}}^{{{\text{1}} \mathord{\left/ {\vphantom {{\text{1}} {\text{3}}}} \right. \kern-\nulldelimiterspace} {\text{3}}}} }} \right)} \) [25] and the Framingham formula derived by linear regression \( {\left[ {{\text{QT}}_{{{\text{c}}\;{\text{Sagie}}}} {\text{ = QT + 0}}{\text{.154}}{\left( {{\text{1 - RR}}} \right)}} \right]} \) were used [26].
Follow-up and outcome definition
The status (alive/dead) and date of death of each subject were ascertained as per 1 January 1998 on the basis of data obtained from population registries. In deceased patients, the underlying cause of each death was determined from a copy of the death certificate, hospital records, post-mortem reports (where available), and additional information given by the treating physicians. Causes of death were coded according to the International Classification of Disease (ICD-9). Cardiovascular mortality included codes 390 to 459 and 798.1, cardiac mortality codes 390 to 429 and 798.1, and mortality due to ischaemic heart disease codes 410 to 414.
Statistical analysis
Statistical assessment of potential differences in baseline characteristics between patients with type 1 and type 2 diabetes mellitus was carried out using the two-tailed unpaired Student’s t test for continuous variables and the Pearson’s chi-squared test for proportions. The impact of QTc interval and rHR on mortality rates was assessed by time-to-event analysis using Cox proportional hazards models. Date of last clinical contact or documented date of leaving Switzerland was used for censored subjects, and exact date of death for subjects who had died. Analyses were conducted separately for subjects with type 1 and type 2 diabetes respectively, regarding all-cause, cardiovascular and cardiac mortality, and death due to ischaemic heart disease. Univariable analyses were performed before adjusting the regression model for age and sex. Then a ‘full model’ was fitted including the following explanatory variables: age, sex, BMI, duration of diabetes, total cholesterol, triacylglycerol, fasting plasma glucose, presence of hypertension/antihypertensive medication, history of coronary heart disease, history of microvascular disease, smoking, alcohol consumption, treatment with insulin (subjects with type 2 diabetes) and treatment with diuretics. QTc was included in the analysis of rHR and vice versa. The model’s assumptions (proportionality) were regularly checked. Analyses were then repeated using cut-off values corresponding to the lower limits of the upper quartiles (QTc interval ≥450 ms or <450 ms in type 1, rHR ≥90 beats per min [bpm] or <90 bpm in type 2 diabetes). Comparable cut-off values have been suggested in earlier reports [13, 27, 28]. Based on these cut-off values, Kaplan–Meier survival analyses were performed for the main endpoint (overall mortality) and differences were statistically assessed using log-rank test. To formally assess effect modification of QTc and rHR by diabetes type a confirmatory analysis was performed including terms of interaction in an analysis by Cox regression. Finally, regression models using the Bazett formula for QTc were compared with models using the formula suggested by Fridericia [25] and by Sagie [26]. All analyses were performed using Stata version 8.2 (Stata Corporation, College Station, TX, USA). Results are given as mean±SD and as hazard ratio (HR) with 95% CI. p values <0.05 were considered statistically significant.
Results
Study characteristics
The entire cohort comprised 533 patients and baseline ECGs were available in 523 patients (221 type 1 diabetes, 302 type 2 diabetes). During follow-up 18 patients left the country and were censored accordingly. This translated into a drop-out rate of 3.4%. Baseline ECG was normal in more than three-quarters of all patients (85% and 77% for types 1 and 2 diabetes, respectively). The mean difference of repeated determinations of QTc was 2.8%. Mean follow-up was 22.6 ± 0.6 years corresponding to a total of 11,815 person-years. Baseline characteristics are shown in Table 1. The proportion of women in the type 1 diabetes group was 54%, that for type 2 diabetes was lower (44%). Overall there were slightly more men than women (278, 255). Subjects with type 1 diabetes were generally younger, but had a longer duration of diabetes and a higher prevalence of retinopathy as well as higher mean values for fasting glucose at baseline. In contrast, subjects with type 2 diabetes showed higher values for BMI, blood pressure, and lipids, with coronary heart disease reported more frequently. Only a minority of subjects with type 2 diabetes were being treated with diet alone; two-thirds were using oral glucose-lowering drugs (e.g. sulfonylureas and/or biguanides), and less than one-third were being treated with insulin. In contrast, all patients with type 1 diabetes used insulin, with a minority also receiving oral glucose-lowering drugs (e.g. biguanides). While the proportion of subjects treated with antihypertensive drugs other than diuretics as well as with lipid-lowering drugs was comparable for the two types of diabetes, the use of diuretics was more frequent in subjects with type 2 diabetes. At baseline rHR tended to be higher and QTc interval was significantly longer in patients with type 1 diabetes than in those with type 2 diabetes.
All-cause mortality
During the study period 107 subjects with type 1 diabetes and 158 subjects with type 2 diabetes died. In subjects with type 1 diabetes, QTc was positively associated with overall mortality. The unadjusted HR was 1.07 per 10 ms increase of QTc (95% CI 1.01–1.15, p = 0.033). This was not substantially altered when the model was adjusted for age and sex (HR 1.10, 95% 1.02–1.18, p = 0.009). The association persisted after additional inclusion of further explanatory variables as stated in Subjects and methods (‘full model’, HR 1.10, 1.02–1.20, p = 0.011). In contrast, no association of rHR with this endpoint was detected in type 1 diabetes (p = 0.924) (Fig. 1, Tables 2 and 3). Subjects with type 2 diabetes revealed a strong positive association between rHR and mortality due to all causes. The unadjusted HR was 1.27 per 10 bpm (95% CI 1.14–1.41, p < 0.001). Again, adjustment for age and sex revealed a similar HR (1.28, 1.16–1.42, p < 0.001), which was not substantially altered in the fully adjusted model (HR 1.31, 1.15–1.50, p < 0.001). A comparable effect was not detected for QTc in these patients (p = 0.380). The analysis of an effect modification of QTc and rHR by diabetes type revealed a statistically significant association between QTc and type 1 diabetes and between rHR and type 2 diabetes (p = 0.026 and p = 0.014 for terms of interaction; Fig. 1), thereby underscoring the differences between the two types of diabetes. Subjects with type 1 diabetes and a QTc ≥450 ms had a twofold increased mortality risk compared with those with a QTc <450 ms (HR 2.04, 95% CI 1.27–3.25, p = 0.003). In type 2 diabetes, a comparable increase in risk was observed when subjects with a rHR ≥90 bpm were compared with those with rHR <90 bpm (HR 2.23, 95% CI 1.43–3.46, p < 0.001). Results of Kaplan–Meier survival analysis are shown in Fig. 2 (p values for log-rank test 0.019 and 0.001 for type 1 and type 2 diabetes, respectively).
Hazard ratios for mortality due to all causes, cardiovascular disease, cardiac disease, and ischaemic heart disease per incremental 10 ms prolongation of QTc interval and per 10 bpm increase in resting heart rate, respectively. p values are for effect modification by type of diabetes (closed circles, type 1 diabetes; open circles, type 2 diabetes)
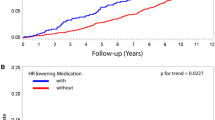
Kaplan–Meier estimation of survival probabilities for overall mortality. a The survival probabilities for type 1 diabetes, comparing patients with a QTc interval ≥450 ms (solid line) with those with a QTc interval <450 ms (dotted line). p = 0.019 for difference by log-rank test. b Survival probabilities for type 2 diabetes comparing patients with a resting heart rate ≥90 bpm (solid line) with those with rHR <90 bpm (dotted line). p = 0.001 for difference by log-rank test
Cardiovascular mortality
In 50 subjects with type 1 and in 76 subjects with type 2 diabetes, death was classified as due to cardiovascular disease. As for all-cause mortality, QTc but not rHR was positively associated with cardiovascular mortality in type 1 diabetes (Fig. 1, Table 2). HRs for QTc tended to be lower in the unadjusted model and after inclusion of age and sex when compared with the fully adjusted model, although conventional levels of significance were reached only in the latter (Table 3). Inverse findings were observed in subjects with type 2 diabetes, where rHR but not QTc was related to this endpoint (Fig. 1, Table 2). Again, the HR in the unadjusted model was similar to those after adjustment for age and sex and to the ‘full model’ (Table 3). Confirmatory analysis using interaction terms revealed that the differences between the two types of diabetes were unlikely to be a chance finding (p = 0.008 and p = 0.008 for interaction, respectively; Fig. 1). In type 1 diabetes, mortality risk for a QTc ≥450 ms was again increased twofold compared with a QTc <450 ms (HR 2.34, 95% CI 1.16–4.71, p = 0.018). In type 2 diabetes a rHR ≥90 bpm was even associated with a threefold increased risk (HR 3.27, 95% CI 1.76–6.09, p < 0.001).
Cardiac mortality
Cardiac mortality was confirmed in 43 type 1 and 71 type 2 diabetic patients. In the former, QTc but not rHR was significantly associated with cardiac mortality (Fig. 1, Table 2). HRs tended to be slightly higher in the fully adjusted model compared with the unadjusted analysis (Table 3). In type 2 diabetes, rHR was found to predict cardiac mortality, whereas QTc was not (Fig. 1, Table 2). Adjustment for age and sex and inclusion of further explanatory variables did not affect HRs (Table 3). Again, effect modification showed that QTc was related to type 1 and rHR to type 2 diabetes (p = 0.006 and p = 0.009 for interaction, respectively; Fig. 1). HR for subjects with type 1 diabetes and QTc ≥450 ms was 2.90 (95% CI 1.36–6.16, p = 0.006). In subjects with type 2 diabetes, risk of cardiac mortality was comparably increased for those with a rHR ≥90 bpm (HR 3.51, 95% CI 1.86–6.64, p < 0.001).
Death due to ischaemic heart disease
There were 25 and 52 deaths due to ischaemic heart disease in subjects with type 1 and type 2 diabetes, respectively. In the former, no statistically significant association was found for either QTc nor rHR (Fig. 1, Tables 2 and 3). In subjects with type 2 diabetes, a strong positive association persisted for rHR but not for QTc (p = 0.010 for interaction; Fig. 1, Tables 2 and 3). Type 2 diabetic subjects with a rHR of ≥90 bpm had a more than threefold increased risk of dying from ischaemic heart disease (HR 3.33, 95% CI 1.63–6.77, p = 0.001).
Different formulas for correcting QT interval for heart rate
Similar results were obtained using the formulas proposed by Fridericia [25] or Sagie [26] when compared with Bazett’s formula [24]. For example, in subjects with type 1 diabetes, HR for all-cause mortality was 1.10 using Bazett’s model. Applying Fridericia’s or Sagie’s approach the corresponding values were 1.12 and 1.13, respectively (Table 2). The same additional explanatory variables were used for all three analyses. The current literature is mainly based on Bazett’s formula. In order to compare the present findings with previous reports, the formula suggested by Bazett was included in the final model.
Discussion
The main finding of this 23-year follow-up was a difference in the prognostic value of rHR and QTc between the two types of diabetes. In subjects with type 1 diabetes QTc, but not rHR was associated with an increased risk of all-cause mortality and mortality due to cardiovascular and cardiac disease. In contrast, in type 2 diabetes rHR, but not QTc was consistently related to mortality due to all causes, cardiovascular, cardiac, and ischaemic heart disease. Interestingly, in type 1 diabetes a QTc interval ≥450 ms translated into a twofold increase in all-cause mortality and a threefold increase in cardiac mortality. In subjects with type 2 diabetes a comparable increase in mortality risk was found for a rHR of ≥90 bpm compared with a rHR <90 bpm.
To our knowledge, this is the first study to prospectively assess the role of QTc and rHR in both types of diabetes within the same study framework. Its findings confirm the prognostic value of QTc as an independent risk factor for all-cause mortality in type 1 diabetes [5, 28–30]. In addition to the results of Rossing et al. [5], the present analysis found QTc to be associated not only with overall mortality but also with cardiovascular and cardiac mortality. Moreover, it also reproduced the findings of Sawicki and colleagues, which were made in subjects with nephropathy [28] in a more general sample of subjects with type 1 diabetes. The HR for overall mortality found in the present analysis was comparable to that in Rossing’s report [5], but tended to be lower than the risk ratio found in subjects with overt nephropathy [28] (1.10 and 1.47 per 10 ms, respectively). Earlier reports have suggested that the association between QTc and cardiovascular disease was stronger in male than in female subjects with type 1 diabetes [30]. The present study had slightly more female subjects in the group with type 1 diabetes, thereby potentially underestimating the prognostic value of QTc for this patient group.
An increased rHR has been found to be related to all-cause mortality and cardiovascular death in several trials of non-diabetic subjects [13, 31–34], and in subjects with type 2 diabetes [8, 10]. Our analysis confirmed the role of rHR as an easily measurable factor for risk assessment of all-cause and cardiovascular mortality in subjects with type 2 diabetes, and had comparable HRs. In addition to findings of previous reports, we also observed a relation between elevated rHR and both cardiac mortality and mortality due to ischaemic heart disease. On the other hand, the present analysis did not confirm an association between QTc and any of the endpoints in type 2 diabetes as has been reported previously [6, 7, 9, 10, 35]. Given previous hypotheses that prolongation of QTc as a marker of cardiac autonomic neuropathy could be of greater importance in type 1 than in type 2 diabetes [5], our findings on this count are intriguing, and it can only be speculated on the underlying mechanisms.
In subjects with type 1 diabetes an increased prevalence of prolonged QTc has been reported before [30, 36], in particular in subjects with autonomic neuropathy [36–38]. This may indicate that increased QTc relates to diabetic autonomic neuropathy [39]. QTc prolongation has been suggested to result from a reduction in vagal activity and an increased sympathetic tonus, thereby reflecting myocardial autonomic instability and an increased risk of arrhythmia and cardiac death [9, 28, 36, 40]. Compared with previous publications reporting an association of QTc with mortality in type 2 diabetes [9, 10, 35], subjects with type 2 diabetes were substantially younger in the present study. It should also be noted that some earlier reports revealing an association between QTc and mortality were cross-sectional [35] or based on a case–control design [9] and in the case of prospective trials [6, 7, 10] had a considerably shorter follow-up.
In type 2 diabetes hyperglycaemia is often associated with a pro-inflammatory state including obesity, dyslipidaemia and hypertension, thereby promoting the development of atherosclerosis [41]. Interestingly, impaired myocardial oxygen supply has also been shown to influence QT interval [28, 30]. As has been pointed out before [6, 10], QTc is possibly a composite marker, reflecting abnormal ventricular repolarisation due to ischaemia, fibrosis, left ventricular hypertrophy and dilatation, autonomic neuropathy, and vascular damage, conditions frequently present in diabetic myocardium. Although the models used in this analysis were adjusted for the pre-existence of coronary heart disease, we could not fully rule out a potential interference due to silent heart disease in subjects with diabetes, since invasive cardiac procedures were not performed.
The strength of the present study lies in the long follow-up period, the well-defined cohort of diabetic subjects (Swiss cohort [17] of the WHO Multinational Study of Vascular Disease in Diabetes [18, 22]), the evaluation of pre-specified endpoints, and the small drop-out rate. Considerable efforts were undertaken to adjust for relevant factors known to affect cardiovascular risk and/or myocardial ventricular repolarisation This allowed inclusion of parameters that earlier reports had not been adjusted for, despite their known influence on mortality (e.g. alcohol consumption) [42]. Moreover, as the use of Bazett’s formula [24] to calculate QTc has been questioned before [8, 26, 43], possible differences in effect were taken into account by performing sensitivity analyses using the formulas suggested by Fridericia [25] and by Sagie [26]. In contrast to earlier reports [8], inclusion of different calculations of QTc did not substantially alter our results (Table 2). As a consequence, Bazett’s formula was included in the final model to allow for comparisons with other reports.
Nevertheless, we must acknowledge some limitations to our findings. Thus rHR was determined from ECG recordings, whereas in clinical practice it is usually measured by pulse palpation, rendering it subject to variation due to circumstantial factors (medical setting, circadian rhythm, body position etc.). In addition, QTc and rHR can potentially be influenced by specific medication. Although the use of antihypertensive medication and diuretics was recorded in the present trial, data did not allow to specifically adjust for the use of beta blockers. Since it is known that treatment with these agents can influence both QTc [44], and rHR, a potential interfering effect on the present findings cannot be fully excluded. It should, however, be noted that at the time of study entry only a limited number of beta blockers was available in Switzerland. Moreover, in another study [9] adjustment for use of beta blockers only modestly attenuated the association between QTc and the risk of primary cardiac arrest. Another known factor to influence rHR is physical training [45]. Tachycardia may be a marker of decreased physical fitness, which in turn may be associated with an increased risk of mortality [46]. Interestingly, increased rHR was found to be an independent prognostic factor of cardiovascular mortality in studies controlling for energy expenditure as an indicator of physical fitness [31].
In deceased patients, the underlying cause of death was determined from a copy of the death certificate, hospital records, post-mortem reports, and additional information given by the treating physicians. Despite intensive efforts to collect comprehensive data, the cause of death may, in some cases, have been misclassified, especially if based only on death certificates, which are a comparatively unreliable source of information. Thus, our findings regarding all-cause mortality are clearly more robust than those for cause-specific mortality.
Glycaemic control has been shown to be related to macrovascular complications [47, 48], and was, therefore, included in the present analysis. Importantly, information on glycaemic control had to be based on fasting glucose, since HbA1c was not available at the time of study entry, thereby potentially limiting the accuracy of adjustment. With regard to this, however, Veglio et al. did not report a significant influence of HbA1c levels on QTc [35].
In summary, this study, performed in a large, diabetic cohort followed over 23 years, confirms that in subjects with type 1 diabetes prolonged QTc is associated with an increased mortality risk due to all causes, and to cardiovascular and cardiac disease, whereas no association was found for rHR. In contrast, in subjects with type 2 diabetes, elevated rHR, but not QTc, is associated with an increased risk of all-cause mortality as well as risk of death due to cardiovascular, cardiac and ischaemic heart disease. The underlying pathophysiological mechanisms are probably complex and remain to be fully elucidated.
Abbreviations
- bpm:
-
beats per min
- HR:
-
hazard ratio
- QTc :
-
QT interval corrected for heart rate
- rHR:
-
resting heart rate
- V:
-
ventral
References
Waernbaum I, Blohme G, Ostman J et al (2006) Excess mortality in incident cases of diabetes mellitus aged 15 to 34 years at diagnosis: a population-based study (DISS) in Sweden. Diabetologia 49:653–659
Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM (2006) All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992–1999. Diabetologia 49:660–666
Nathan DM, Cleary PA, Backlund JY et al (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353:2643–2653
Ford ES, DeStefano F (1991) Risk factors for mortality from all causes and from coronary heart disease among persons with diabetes. Findings from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Am J Epidemiol 133:1220–1230
Rossing P, Breum L, Major-Pedersen A et al (2001) Prolonged QTc interval predicts mortality in patients with Type 1 diabetes mellitus. Diabet Med 18:199–205
Naas AA, Davidson NC, Thompson C et al (1998) QT and QTc dispersion are accurate predictors of cardiac death in newly diagnosed non-insulin dependent diabetes: cohort study. Br Med J 316:745–746
Christensen PK, Gall MA, Major-Pedersen A et al (2000) QTc interval length and QT dispersion as predictors of mortality in patients with non-insulin-dependent diabetes. Scand J Clin Lab Invest 60:323–332
Linnemann B, Janka HU (2003) Prolonged QTc interval and elevated heart rate identify the type 2 diabetic patient at high risk for cardiovascular death. The Bremen Diabetes Study. Exp Clin Endocrinol Diabetes 111:215–222
Whitsel EA, Boyko EJ, Rautaharju PM et al (2005) Electrocardiographic QT interval prolongation and risk of primary cardiac arrest in diabetic patients. Diabetes Care 28:2045–2047
Cardoso CR, Salles GF, Deccache W (2003) Prognostic value of QT interval parameters in type 2 diabetes mellitus: results of a long-term follow-up prospective study. J Diabetes Complications 17:169–178
Rana BS, Band MM, Ogston S, Morris AD, Pringle SD, Struthers AD (2002) Relation of QT interval dispersion to the number of different cardiac abnormalities in diabetes mellitus. Am J Cardiol 90:483–487
Sawicki PT, Kiwitt S, Bender R, Berger M (1998) The value of QT interval dispersion for identification of total mortality risk in non-insulin-dependent diabetes mellitus. J Intern Med 243:49–56
Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA (1987) Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 113:1489–1494
Palatini P, Julius S (1997) Heart rate and the cardiovascular risk. J Hypertens 15:3–17
Palatini P, Casiglia E, Julius S, Pessina AC (1999) High heart rate: a risk factor for cardiovascular death in elderly men. Arch Intern Med 159:585–592
Mensink GB, Hoffmeister H (1997) The relationship between resting heart rate and all-cause, cardiovascular and cancer mortality. Eur Heart J 18:1404–1410
Teuscher A, Herman JB, Studer PP (1983) Vascular diseases in 534 Swiss diabetics within the scope of a multinational study. Klin Wochenschr 61:139–149 (in German)
Jarrett RJ, Keen H, Grabauskas V (1979) The WHO multinational study of vascular disease in diabetes: 1. General description. Diabetes Care 2:175–186
Lee ET, Keen H, Bennett PH, Fuller JH, Lu M (2001) Follow-up of the WHO Multinational Study of Vascular Disease in Diabetes: general description and morbidity. Diabetologia 44(Suppl 2):S3–S13
Fuller JH, Stevens LK, Wang SL (2001) Risk factors for cardiovascular mortality and morbidity: the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 44(Suppl 2):S54–S64
Miki E, Lu M, Lee ET, Keen H, Bennett PH, Russell D (2001) The incidence of visual impairment and its determinants in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 44(Suppl 2):S31–S36
No authors listed (1985) Prevalence of small vessel and large vessel disease in diabetic patients from 14 centres. The World Health Organisation Multinational Study of Vascular Disease in Diabetics. Diabetes Drafting Group. Diabetologia 28(Suppl):615–640
Cowan JC, Hilton CJ, Griffiths CJ et al (1988) Sequence of epicardial repolarisation and configuration of the T wave. Br Heart J 60:424–433
Bazett HC (1920) An analysis of time relation of electrocardiograms. Heart 7:353–370
Fridericia LS (1920) Die Systolendauer im Elektrokardiogramm bei normalen Menschen und bei Herzkranken. Acta Med Scand 53:469–486
Sagie A, Larson MG, Goldberg RJ, Bengtson JR, Levy D (1992) An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol 70:797–801
Fujiura Y, Adachi H, Tsuruta M, Jacobs DR Jr, Hirai Y, Imaizumi T (2001) Heart rate and mortality in a Japanese general population: an 18-year follow-up study. J Clin Epidemiol 54:495–500
Sawicki PT, Dahne R, Bender R, Berger M (1996) Prolonged QT interval as a predictor of mortality in diabetic nephropathy. Diabetologia 39:77–81
Veglio M, Sivieri R, Chinaglia A, Scaglione L Cavallo-Perin P (2000) QT interval prolongation and mortality in type 1 diabetic patients: a 5-year cohort prospective study. Neuropathy Study Group of the Italian Society of the Study of Diabetes, Piemonte Affiliate. Diabetes Care 23:1381–1383
Veglio M, Borra M, Stevens LK, Fuller JH, Perin PC (1999) The relation between QTc interval prolongation and diabetic complications. The EURODIAB IDDM Complication Study Group. Diabetologia 42:68–75
Gillum RF, Makuc DM, Feldman JJ (1991) Pulse rate, coronary heart disease, and death: the NHANES I Epidemiologic Follow-up Study. Am Heart J 121:172–177
Fuller JH, McCartney P, Jarrett RJ et al (1979) Hyperglycaemia and coronary heart disease: the Whitehall study. J Chronic Dis 32:721–728
Dyer AR, Persky V, Stamler J et al (1980) Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol 112:736–749
Filipovsky J, Ducimetiere P, Safar ME (1992) Prognostic significance of exercise blood pressure and heart rate in middle-aged men. Hypertension 20:333–339
Veglio M, Bruno G, Borra M et al (2002) Prevalence of increased QT interval duration and dispersion in type 2 diabetic patients and its relationship with coronary heart disease: a population-based cohort. J Intern Med 251:317–324
Sivieri R, Veglio M, Chinaglia A, Scaglione P, Cavallo-Perin P (1993) Prevalence of QT prolongation in a type 1 diabetic population and its association with autonomic neuropathy. The Neuropathy Study Group of the Italian Society for the Study of Diabetes. Diabet Med 10:920–924
Ewing DJ, Boland O, Neilson JM, Cho CG, Clarke BF (1991) Autonomic neuropathy, QT interval lengthening, and unexpected deaths in male diabetic patients. Diabetologia 34:182–185
Veglio M, Chinaglia A, Borra M, Perin PC (1995) Does abnormal QT interval prolongation reflect autonomic dysfunction in diabetic patients? QTc interval measure versus standardized tests in diabetic autonomic neuropathy. Diabet Med 12:302–306
Vinik AI, Maser RE, Mitchell BD, Freeman R (2003) Diabetic autonomic neuropathy. Diabetes Care 26:1553–1579
Ewing DJ, Neilson JM (1990) QT interval length and diabetic autonomic neuropathy. Diabet Med 7:23–26
Schwartz CJ, Valente AJ, Sprague EA, Kelley JL, Cayatte AJ, Rozek MM (1992) Pathogenesis of the atherosclerotic lesion. Implications for diabetes mellitus. Diabetes Care 15:1156–1167
Diem P, Deplazes M, Fajfr R et al (2003) Effects of alcohol consumption on mortality in patients with Type 2 diabetes mellitus. Diabetologia 46:1581–1585
Ahnve S (1991) Is QT interval prolongation a strong or weak predictor for cardiac death? Circulation 84:1862–1865
Molgaard H, Mickley H, Pless P, Bjerregaard P, Moller M (1993) Effects of metoprolol on heart rate variability in survivors of acute myocardial infarction. Am J Cardiol 71:1357–1359
Somers VK, Conway J, Johnston J, Sleight P (1991) Effects of endurance training on baroreflex sensitivity and blood pressure in borderline hypertension. Lancet 337:1363–1368
Palatini P (1999) Need for a revision of the normal limits of resting heart rate. Hypertension 33:622–625
Selvin E, Marinopoulos S, Berkenblit G et al (2004) Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 141:421–431
Stettler C, Allemann S, Jüni P et al (2006) Glycemic control and macrovascular disease in type 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J 152:27–38
Acknowledgements
We are indebted to the study participants, their treating physicians, and to the Federal Office of Statistics, Neuchatel (Th. Spuler), as well as to the various community-based population registries throughout the country. We thank E. Hurni, R. Fajfr, P. P. Studer, H. Schnell, St. Suter and K. Diem for help with data collection and verification.
Duality of interest
The authors declare that they have no duality of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stettler, C., Bearth, A., Allemann, S. et al. QTc interval and resting heart rate as long-term predictors of mortality in type 1 and type 2 diabetes mellitus: a 23-year follow-up. Diabetologia 50, 186–194 (2007). https://doi.org/10.1007/s00125-006-0483-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-006-0483-1