Abstract
Aims/hypothesis
Disturbances in substrate source metabolism and, more particularly, in fatty acid metabolism, play an important role in the aetiology and progression of type 2 diabetes. However, data on substrate source utilisation in type 2 diabetes are inconclusive.
Methods
[U-13C]palmitate and [6,6-2H2]glucose tracers were used to assess plasma NEFA and glucose oxidation rates and to estimate the use of muscle- and/or lipoprotein-derived triacylglycerol and muscle glycogen. Subjects were ten male patients who had a long-term (7 ± 1 years) diagnosis of type 2 diabetes and were overweight, and ten matched healthy, male control subjects. Muscle biopsy samples were collected before and after exercise to assess muscle fibre type-specific intramyocellular lipid and glycogen content.
Results
At rest and during exercise, the diabetes patients had greater values than the controls for palmitate rate of appearance (Ra) (rest, 2.46 ± 0.18 and 1.85 ± 0.20 respectively; exercise, 3.71 ± 0.36 and 2.84 ± 0.20 μmol kg−1 min−1) and rate of disappearance (Rd) (rest, 2.45 ± 0.18 and 1.83 ± 0.20; exercise, 3.64 ± 0.35 and 2.80 ± 0.20 μmol kg−1 min−1 respectively). This was accompanied by significantly higher fat oxidation rates at rest and during recovery in the diabetes patients (rest, 0.11 ± 0.01 in diabetes patients and 0.09 ± 0.01 in controls; recovery, 0.13 ± 0.01 and 0.11 ± 0.01 g/min respectively), despite significantly greater plasma glucose Ra, Rd and circulating plasma glucose concentrations. Furthermore, exercise significantly lowered plasma glucose concentrations in the diabetes patients, as a result of increased blood glucose disposal.
Conclusion
This study demonstrates that substrate source utilisation in long-term-diagnosed type 2 diabetes patients, in whom compensatory hyperinsulinaemia is no longer present, shifts towards an increase in whole-body fat oxidation rate and is accompanied by disturbances in fat and carbohydrate handling.
Similar content being viewed by others
Introduction
Disturbances in fatty acid metabolism, as found in sedentary, obese and/or type 2 diabetes patients, are an important factor in the development of skeletal muscle insulin resistance [1–3]. Elevated fatty acid delivery and/or impaired fatty acid oxidation result in net intramyocellular accumulation of triacylglycerol and fatty acid metabolites (such as fatty acyl-CoA, ceramides and diacylglycerol). The latter are likely to induce defects in the insulin signalling cascade, thus causing insulin resistance [4–10] in skeletal muscle.
On a whole-body level, conflicting data exist on the proposed disturbances in substrate metabolism in type 2 diabetes patients. In these patients, basal oxidation rates of whole-body total fat have been reported to be both increased [11–13] and similar [14–19] compared with lean [11–13] and overweight/obese [14–19] controls respectively. Whole-body plasma NEFA uptake and/or oxidation rates at rest have been reported to be either similar [17, 18] or decreased [16] in the type 2 diabetes patient. However, data on arteriovenous differences in plasma NEFA concentrations over the leg or arm have more consistently displayed reduced fasting plasma NEFA uptake and/or oxidation in type 2 diabetes patients compared with normoglycaemic controls [11, 14, 20], despite elevated systemic plasma NEFA and glucose levels [14, 20]. During exercise, in both the prediabetes and/or type 2 diabetes state, the uptake and oxidation of plasma NEFA and other lipid sources have been shown to be similar [17, 18] or decreased [16, 21]. A cross-sectional review of the literature on the use of lipoprotein and/or muscle-derived triacylglycerol suggests that type 2 diabetes patients have a reduced capacity to mobilise and/or oxidise the intramyocellular lipid stores [22]. However, a direct comparison of intramyocellular triacylglycerol (IMTG) use between diabetes patients and matched controls has not yet been made.
Exercise can lower plasma glucose concentrations in type 2 diabetes patients. This can be attributed to a blunted increase in hepatic glucose output [23] and/or an increase in whole-body glucose uptake rate [15, 17, 18, 24]. The latter has been shown to result in greater carbohydrate oxidation rates during exercise in type 2 diabetes patients in some [24] but not all studies [15–18]. Data on differences in substrate source utilisation during post-exercise recovery between diabetes patients and healthy, normoglycaemic controls are entirely lacking in the literature.
The apparently inconsistent findings on aberrations in whole-body substrate utilisation in the type 2 diabetes state can be explained by differences in the methods used and in the selected subpopulations of type 2 diabetes patients and controls. These equivocal data restrict conclusive insight into the exact nature and extent of the metabolic disturbances that play a key role in the aetiology and progression of type 2 diabetes. In the present study, we investigated the disturbances in whole-body substrate source utilisation in long-standing type 2 diabetes at rest, during exercise and subsequent recovery by using contemporary stable isotope methods combined with skeletal muscle biopsy sampling. Patients with long-standing type 2 diabetes were selected to assess the metabolic disturbances when compensatory hyperinsulinaemia is no longer present. Careful matching of the type 2 diabetes patients with normoglycaemic controls was performed to be able to determine the effect of type 2 diabetes on substrate use independently of age, body composition and aerobic capacity. The present study is the first to provide a complete overview of substrate source utilisation rates at rest, during exercise and post-exercise recovery in overweight patients with long-standing type 2 diabetes, in whom compensatory hyperinsulinaemia is no longer present.
Subjects and methods
Subjects
Ten male, sedentary, overweight type 2 diabetes patients and ten male, sedentary, weight-matched healthy controls (Table 1) participated in this study. All patients were using metformin with (n=7) or without (n=3) sulphonylurea derivatives (gliclazide, glimepiride or tolbutamide). Exclusion criteria were impaired liver function, renal failure and/or a history of severe cardiovascular problems. Diabetic status was verified with an OGTT according to WHO criteria [25]. Medication was withheld for 24 h prior to the trials. Subjects were informed about the nature and risks of the experimental procedures before their written informed consent was obtained. The study was performed according to the principles of the Declaration of Helsinki and was approved by the local medical ethical committee.
Pretesting
Maximal workload capacity (Wmax) and maximal oxygen uptake capacity (VO2max) were determined with an electronically braked cycle ergometer (Excalibur, Lode, Groningen, the Netherlands) during an incremental exhaustive exercise test 2 weeks prior to the first trial [17]. Oxygen uptake (VO2) and carbon dioxide production (VCO2) were measured with an Oxycon β (Mijnhart, Breda, the Netherlands). Body composition was assessed using the hydrostatic weighing method. Body fat percentage was calculated using Siri’s equation [26].
Diet and physical activity prior to testing
All subjects were instructed to refrain from strenuous physical activity for 2 days prior to each trial. In addition, they recorded dietary intake during 2 days prior to the first trial and repeated this diet prior to the second trial. The evening before each trial, all subjects received the same standardised meal (42 kJ [10 kcal]/kg; consisting of 61% of energy as carbohydrate, 24% as fat and 15% as protein). There were no differences between groups in daily energy intake and macronutrient composition of the diet.
Experimental trials
Each subject performed one main trial and an additional test to determine the acetate recovery factor. Both tests were separated by at least 1 week. Each trial consisted of 120 min of resting measurements followed by 60 min of cycling exercise at an exercise intensity set at 50% Wmax, and a subsequent 120 min recovery period. In the main trial, a [U-13C]palmitate and [6,6-2H2]glucose tracer was infused and breath, blood and muscle samples were collected at regular intervals. The acetate test was identical with the exception of the infusion of [1,2-13C]acetate and the collection of breath samples only [27].
Protocol
After an overnight fast, subjects arrived at the laboratory at 08.00 hours by car or public transport. After 30 min of supine rest, a percutaneous muscle biopsy [28] was taken from the vastus lateralis muscle. A Teflon catheter (Baxter, Utrecht, the Netherlands) was inserted into an antecubital vein of one arm for blood sampling and another catheter was inserted in the contralateral arm for isotope infusion. Subsequently, subjects were administered a single intravenous dose of NaH13CO3 (0.06375 mg/kg), followed by a [6,6-2H2]glucose prime (13.5 μmol/kg). Thereafter, a continuous infusion of [6,6-2H2]glucose (0.3 μmol kg−1 min−1) and [U-13C]palmitate (0.01 μmol kg−1 min−1) (or [1,2-13C]acetate) was started (t = 0 min) via a calibrated IVAC pump (IVAC 560; Ivac, San Diego, CA, USA). At t = 120 min subjects started to exercise on a cycle ergometer at 50% Wmax for a 60 min period. Whilst the subject was at rest, VO2 and VCO2 were measured from t = 60 to 120 min (Oxycon-β; Mijnhart); during exercise VO2 and VCO2 were measured for 5 min every 15 min prior to sampling of blood and expired breath. Immediately after cessation of exercise, a second muscle biopsy was taken, after which the subject rested supine for 2 h. VO2 and VCO2 were measured during recovery from t = 210 to 270 min.
Blood and breath samples
Blood samples (7 ml) were collected in EDTA-containing tubes and centrifuged at 1,000 g for 10 min at 4°C. Aliquots of plasma were frozen immediately in liquid nitrogen and stored at −80°C. Plasma concentrations of glucose (Roche, Basel, Switzerland), lactate, NEFA (Wako Chemicals, Neuss, Germany), glycerol (Roche Diagnostics, Indianapolis, IN, USA) and triacylglycerol (Sigma Diagnostics, St Louis, MO, USA) were analysed with a COBAS semi-automatic analyser (Roche). Plasma insulin was measured by radioimmunoassay (Linco, St Charles, MO, USA). Blood HbA1c content was analysed by high-performance liquid chromatography (Bio-Rad Diamat, Munich, Germany). Expired breath samples were analysed for 13C/12C ratio using a gas chromatograph−isotope ratio mass spectrometer system (GC-IRMS; Finnigan MAT 252, Thermo Electron Corp., Bremen, Germany). For determination of plasma palmitate and NEFA kinetics, NEFA were extracted, isolated by thin-layer chromatography and derivatised to their methyl esters. Palmitate concentration was determined on an analytical GC with flame ionisation detection using heptadecanoic acid as internal standard, and constituted 21.3 ± 0.7% of total NEFA. The isotope tracer/tracee ratio (TTR) of [U-13C]palmitate was determined using GC combustion isotope ratio MS (Finnigan MAT 252). Following derivatisation, plasma [6,6-2H2]glucose enrichment was determined by electron ionisation GC-MS (Finnigan). Palmitate, glucose and acetate tracer concentrations in the infusates averaged 1.05 ± 0.01, 34.4 ± 0.9 and 4.92 ± 0.03 mmol/l respectively. Therefore, the exact tracer infusion rates averaged 9.1 ± 0.1, 277 ± 2 and 75 ± 1 nmol kg−1 min−1 respectively.
Calculations
From respiratory measurements, total fat and carbohydrate oxidation rates were calculated using the non-protein respiratory quotient [29]:
where VO2 and VCO2 are in l/min and oxidation rates in g/min. Rate of appearance (Ra) and rate of disappearance (Rd) of palmitate and glucose were calculated using the single-pool non-steady state Steele equations adapted for stable isotope methodology [30]:
where F is the infusion rate (μmol kg−1 min−1), V is the distribution volume for palmitate or glucose (40 and 160 ml/kg, respectively), C 1 and C 2 are palmitate or glucose concentrations (mmol/l) at times 1 (t 1) and 2 (t 2) respectively, and E 2 and E 1 are the plasma palmitate or glucose enrichments (TTR) at times 1 and 2, respectively. Production of 13CO2 (Pr13CO2; mol/min) from the infused palmitate tracer was calculated as:
where TTRCO2 is the breath 13C/12C ratio at a given time point, VCO2 is carbon dioxide production (l/min), k is the volume of 1 mole of CO2 (22.4 l/mol), and Ar is the fractional 13C label recovery in breath CO2 observed after the infusion of labelled acetate [27, 31, 32], calculated as:
where F is the infusion rate of [1,2-13C] acetate (mol/min). Plasma palmitate oxidation (Rox) (mol/min) can subsequently be calculated as:
where Rd palmitate is the rate of disappearance of palmitate (mol/min), F is the palmitate infusion rate (mol/min) and 16 is the number of labelled carbon atoms in palmitate. Total plasma NEFA oxidation was calculated by dividing palmitate oxidation rates by the fractional contribution of plasma palmitate to total plasma NEFA concentration. The contribution of fat sources other than plasma NEFA was calculated by subtracting plasma NEFA oxidation from total fat oxidation.
In a previous study it has been shown that during exercise (50% Wmax) plasma glucose Rd equals its Rox (96–100%) [33]. Therefore, plasma glucose oxidation rate during exercise was estimated as:
Whole-body muscle glycogen use was calculated by subtracting plasma glucose oxidation from total carbohydrate oxidation. As plasma glucose Rd does not match Rox during resting conditions [34], plasma glucose oxidation rates cannot be calculated accurately at rest when using a [6,6-2H2]glucose tracer.
Muscle sample analysis
Muscle samples were dissected, freed from any visible non-muscle material, frozen in nitrogen-cooled isopentane and embedded in Tissue-Tek (Sakura, Zoeterwoude, the Netherlands). Multiple serial sections (5 μm) were thaw-mounted together on uncoated, precleaned glass slides for each subject. To determine muscle fibre type-specific IMTG content, cross-sections were stained with Oil red O together with immunolabelled cellular constituents using a protocol described before [2]. For each muscle biopsy a total of 58 ± 7 and 49 ± 4 muscle fibres were analysed for lipid content for diabetes patients and control subjects respectively. To permit quantification of intramyocellular glycogen we used the modified PAS stain [35]. For each muscle biopsy, 148 ± 15 and 157 ± 13 muscle fibres were analysed for glycogen content in diabetes and control subjects respectively. Large microscopic overviews containing 167 ± 24 and 174 ± 20 fibres per subject (diabetes and control subjects respectively) were used to determine muscle fibre type composition.
Statistics
All data are expressed as mean ± SEM. To compare tracer kinetics, substrate utilisation rates, IMTG contents and/or plasma metabolite concentrations over time, repeated measures ANOVA was applied. A Scheffé post hoc test was applied in case of a significant F ratio, to locate specific differences. To determine differences between diabetes patients and control subjects, Student’s t test for unpaired observations was used. Simple linear regression was used to investigate specific correlations. Significance was set at the 0.05 level of confidence.
Results
Tracer kinetics
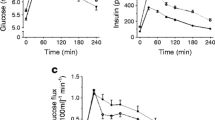
Plasma palmitate and glucose Ra, Rd and Rox are shown in Fig. 1 and Table 2. As plasma palmitate and glucose concentrations were subject to changes throughout rest, exercise and/or recovery (Fig. 2), non-steady-state Steele equations were applied. Table 3 shows breath 13CO2 enrichment and plasma palmitate and glucose enrichment throughout the test. At rest, plasma palmitate Ra, Rd and Rox were stable, and significantly higher in the diabetes patients compared with the controls (Ra and Rd, p < 0.05; Rox, p = 0.07). During exercise, plasma palmitate Ra, Rd and Rox increased over time in both groups (p < 0.01) and were higher in the diabetes group than the control group (Ra and Rd, p < 0.05; Rox, p = 0.08). During post-exercise recovery, plasma palmitate Ra, Rd and Rox were similar between groups. Acetate recovery factors in the diabetes and control groups respectively averaged 0.11 ± 0.00 and 0.13 ± 0.00 at rest, 0.87 ± 0.00 and 0.91 ± 0.00 during exercise, and 0.24 ± 0.00 and 0.26 ± 0.00 during subsequent recovery (no significant difference between groups).
a Plasma rates of appearance (Ra), disappearance (Rd) and oxidation (Rox) of palmitate at rest and during exercise and post-exercise recovery (μmol kg−1 min−1) in the diabetes group (closed symbols) and the control group (open symbols). Circles, Ra; triangles, Rd; squares, Rox. b Plasma glucose rates of appearance (Ra), disappearance (Rd) and oxidation (Rox) at rest and during exercise and post-exercise recovery (μmol kg−1 min−1). Data are mean ± SEM (n = 20). *Ra and Rd significantly different from control group (p < 0.05). Vertical dotted lines mark the beginning and end of exercise
Plasma concentrations of triacylglycerol (a), glucose (b), NEFA (c), non-esterified glycerol (d), lactate (e) and insulin (f) in the diabetes (closed circles) and control groups (open circles). Values are mean ± SEM. *Significantly different from control group (p < 0.05). Vertical dotted lines mark the beginning and end of exercise
At rest, plasma glucose Ra and Rd were stable, and significantly higher in the diabetes patients (p < 0.05). During exercise, plasma glucose Ra and Rd increased over time in both groups (p < 0.01) and were similar between groups. During post-exercise recovery, plasma glucose Ra and Rd were higher in the diabetes patients (p < 0.05). No changes over time in plasma glucose Ra or Rd were observed. In the diabetes patients, the percentage of plasma glucose Ra that disappeared (%Ra/Rd) was significantly greater during exercise than with resting conditions. Furthermore, during exercise, %Ra/Rd was significantly greater in the diabetes group than in the control group.
Substrate utilisation
Total energy expenditure and substrate source utilisation rates are illustrated in Fig. 3. Energy expenditure averaged 5.75 ± 0.22 and 5.28 ± 0.16 kJ/min in the diabetes and control groups respectively. Total fat oxidation rates (g/min) at rest were significantly higher in the diabetes group than in the control group (0.11 ± 0.01 and 0.09 ± 0.01 g/min respectively), contributing 77 ± 2 and 67 ± 3% to total energy expenditure (p < 0.01). The difference in fat oxidation rate was attributed to a significantly higher NEFA oxidation rate in the diabetes group (p < 0.05), with no differences in muscle- and/or lipoprotein-derived triacylglycerol utilisation. The latter contributed only −2 ± 7 and 6 ± 7% to total energy expenditure in the diabetes and control groups respectively.
Whole-body substrate source utilisation at rest (a) and during exercise (b) and post-exercise recovery (c). Black (a–c), plasma NEFA; white (a–c), muscle and lipoprotein-derived triacylglycerol; grey, carbohydrate in a and c, plasma glucose in b; hatching (b), muscle glycogen. *Substrate source oxidation significantly different from control group (p < 0.05); †total fat oxidation significantly different from control group (p < 0.05)
Exercise was performed at a 50% Wmax workload, which resulted in an absolute workload set at 100 ± 7 and 101 ± 8 W (NS) or a relative workload of 63 ± 2% and 54 ± 2 of VO2max (p < 0.05) in the diabetes and control groups, respectively. Energy expenditure during exercise averaged 39 ± 2 and 36 ± 2 kJ/min, respectively. Fat oxidation contributed 42 ± 3 (0.40 ± 0.04 g/min) and 39 ± 3% (0.35 ± 0.03 g/min) to total energy expenditure in the diabetes and control groups, respectively. Muscle- and/or lipoprotein-derived triacylglycerol use increased significantly during exercise conditions to 0.09 ± 0.03 and 0.10 ± 0.02 g/min in the diabetes and control groups respectively, contributing 8 ± 3 and 11 ± 3%, respectively.
Total carbohydrate oxidation rates during exercise averaged 1.37 ± 0.09 and 1.37 ± 0.08 g/min in the diabetes and control groups respectively, contributing 58 ± 3 and 61 ± 3% to total energy expenditure. In the diabetes and control groups, plasma glucose oxidation averaged 0.35 ± 0.03 and 0.32 ± 0.02 g/min respectively, contributing 15 ± 1 and 14 ± 1% to total energy expenditure, with muscle glycogen contributing 43 ± 3 and 46 ± 3% to energy expenditure. Aside from a tendency to greater plasma NEFA oxidation rates in the diabetes group (p = 0.08), no significant differences in substrate source utilisation rates were observed between groups.
During post-exercise recovery, energy expenditure averaged 5.9 ± 0.2 in the diabetes group and 5.5 ± 0.2 kJ/min in the control group, fat oxidation contributing 90 ± 2% (0.13 ± 0.01 g/min) and 80 ± 3% (0.11 ± 0.01 g/min; p < 0.05) to total energy expenditure, respectively. The greater fat oxidation rate in the diabetes group was attributed mainly to a greater plasma NEFA oxidation rate in the diabetes group (p = 0.10), whereas no differences were observed in muscle- and/or lipoprotein-derived triacylglycerol. The greater fat oxidation rates were matched by lower total carbohydrate oxidation rates.
Plasma metabolite concentrations
Plasma metabolite concentrations are shown in Fig. 2. Plasma glucose concentrations were significantly higher in the diabetes group throughout the experiment. During exercise, there was a significant decline in plasma glucose concentration in the diabetes group only. Plasma NEFA concentrations at rest were higher in the diabetes group than the control group. No differences in plasma NEFA between groups were observed during exercise and recovery conditions. Glycerol concentrations increased significantly during rest and exercise and decreased during recovery. Free glycerol concentration tended to be higher in the diabetes group (p = 0.06). Plasma triacylglycerol concentrations declined significantly at rest and during exercise in the diabetes patients, but were stable in control subjects. Plasma insulin levels declined during exercise in both groups (p < 0.01), resulting in significantly lower insulin concentrations during recovery compared with resting levels. No significant differences in plasma insulin concentrations were observed between groups.
Muscle tissue analysis
Muscle fibre type analysis showed 46 ± 3% type I muscle fibres and 54 ± 3% type II fibres in the diabetes group, and 49 ± 4% type I muscle fibres and 51 ± 4% type II fibres in the control group. In both groups, type I fibres contained two to three times more IMTG than type 2 fibres (p < 0.005). Type I and II muscle fibre lipid content averaged 24 ± 4 and 11 ± 2 arbitrary units (AU) respectively in the diabetes group and 34 ± 5 and 11 ± 2 AU respectively in the control group. No significant differences in type I or II muscle fibre lipid content were observed between groups. No significant net changes in type I or type II intramyocellular lipid content were observed following exercise in the diabetes or control group. In the diabetes and control groups, net changes were 8 ± 4 and −6 ± 6 AU respectively for type I fibres and 4 ± 3 and 3 ± 4 for type II fibres (all p > 0.05), respectively. Type I and II muscle fibre glycogen content averaged 36 ± 6 and 32 ± 4 AU respectively in the diabetes group and 36 ± 4 and 46 ± 6 AU in the control group. No significant differences in type I or II muscle fibre glycogen content were observed between groups. After exercise, type II fibres contained significantly more glycogen than type I fibre type in both groups. However, no significant net changes in type I and II muscle fibre glycogen content (type I fibres, −13 ± 6 AU in diabetes group and −6 ± 3 AU in control group; type II fibres, 9 ± 6 AU in diabetes group and −6 ± 5 AU in control group; all p > 0.05) were observed following exercise in both groups.
Discussion
The present study investigated the nature and extent of disturbances in different components of whole-body carbohydrate and fat metabolism in long-term-diagnosed type 2 diabetes patients. Using contemporary stable isotope methods, we found that basal whole-body fat oxidation rates were significantly greater in long-standing type 2 diabetes patients when compared with normoglycaemic controls matched for age, body composition and whole-body oxidative capacity (Fig. 3). This is attributed to significantly higher plasma NEFA appearance rates, resulting in greater NEFA uptake and oxidation rates (Fig. 1). In contrast, resting total carbohydrate oxidation rates were reduced in type 2 diabetes patients, despite substantially elevated plasma glucose appearance rates and the prevalence of hyperglycaemia. Exercise significantly reduces plasma glucose concentrations in type 2 diabetes patients, as glucose disposal exceeds its rate of appearance during exercise conditions.
Fasting whole-body fat oxidation rates were greater in the diabetes patients. This seems to be in contrast to some [14–19], but not all [12, 13, 36], previous studies, which describe normal and/or decreased basal whole-body fatty acid oxidation rates in the type 2 diabetic state [14–19]. These apparently contradictory findings are explained by the fact that long-term-diagnosed diabetes patients were selected to participate in the present study. These patients differ significantly in metabolic profile from their recently diagnosed counterparts in that they no longer show any compensatory hyperinsulinaemia (Fig. 2, Table 1). As a consequence, plasma insulin levels do not compensate for the adipose tissue insulin resistance present in type 2 diabetes [37–40] and fail to inhibit adipose tissue lipolysis. This explains the greater plasma NEFA appearance rates and elevated plasma NEFA availability observed in the diabetes patients (Figs. 1 and 2). The elevated plasma NEFA availability drives a greater NEFA uptake rate and subsequently augments plasma NEFA oxidation in the diabetes patients. The higher basal plasma NEFA turnover and fat oxidation in the diabetes patients were accompanied by a lower carbohydrate oxidation rate (Fig. 3), despite substantially greater hepatic glucose output (Ra glucose) and the presence of marked hyperglycaemia. This clearly demonstrates that carbohydrate metabolism is significantly impaired in the long-term-diagnosed type 2 diabetes patient, and suggests that this might (in part) be attributed to the increased fat oxidation. Interestingly, despite previous studies suggesting substantial differences in the use of plasma glucose, glycogen, NEFA and/or IMTG as a substrate source during exercise conditions in type 2 diabetes patients and normoglycaemic controls [16–18, 24], substrate source utilisation rates were remarkably similar between type 2 diabetes patients and normoglycaemic controls (Fig. 3). However, it should be noted that in the type 2 diabetic state, such apparently normal substrate utilisation rates are observed in the presence of a significantly greater NEFA turnover.
Exercise significantly lowered blood glucose concentrations in type 2 diabetes patients. Though this has been observed before [15, 17, 18, 23, 41], the present study shows that this can be attributed to an improved plasma glucose disposal rate, with plasma glucose Rd representing 118 ± 6% of its Ra during exercise (Table 2). The exercise-induced decline in blood glucose concentrations continued during subsequent recovery, blood glucose Rd remaining well above its Ra (114 ± 1%). These data extend the observations made in previous studies [17, 23, 24, 42] and demonstrate that exercise forms a potent strategy to acutely improve glycaemic control in type 2 diabetes.
Our data do not support the hypothesis that the capacity to mobilise and/or oxidise the IMTG pool is substantially impaired in type 2 diabetes patients when compared with normoglycaemic controls, as suggested previously [2, 43, 44]. Tracer kinetic data (Fig. 1, Table 2) as well as histochemical analysis indicate that muscle (and/or lipoprotein)-derived triacylglycerol play only a relatively minor (quantitative) role as a substrate source at rest and/or during exercise conditions in both sedentary type 2 diabetes patients and healthy sedentary men.
During post-exercise recovery, total fat oxidation rates were elevated compared with pre-exercise resting values in both the type 2 diabetes patients and the normoglycaemic controls (Fig. 3). Furthermore, total fat oxidation rates were greater in the type 2 diabetes patients compared with controls. In contrast, carbohydrate oxidation rates were reduced, which was accompanied by a decreased plasma glucose Ra and Rd.
The present data are not necessarily in contrast to previous findings, which generally show reduced NEFA uptake and/or oxidation rates over the leg or arm in recently diagnosed type 2 diabetes patients [11, 14, 16, 20, 45]. The greater whole-body NEFA turnover rate in long-term-diagnosed type 2 diabetes patients observed in the present study is most likely attributable to the presence of adipose tissue insulin resistance and the absence of compensating hyperinsulinaemia in the selected patient group. Disturbances in whole-body NEFA metabolism remain evident, since the higher total fat oxidation rates occurred under conditions of disproportionally elevated plasma NEFA appearance rates and concomitantly elevated plasma NEFA concentrations. Disturbances in glucose handling in these long-standing diabetes patients are more clearly visible, as total glucose oxidation rates are reduced, despite major upregulation of plasma glucose Ra and Rd and the concomitant hyperglycaemic state. Gaining more insight into the differences in disturbances of substrate utilisation between subpopulations of type 2 diabetes patients (i.e. recently or long-term-diagnosed) will be of great importance to our understanding of the aetiology and progression of the disease.
In conclusion, fasting whole-body fat oxidation rates are elevated in long-term-diagnosed type 2 diabetes patients, which is attributed to greater plasma NEFA appearance rates, elevated plasma NEFA availability, and increased plasma NEFA disappearance and oxidation rates. The greater plasma NEFA appearance rate is most likely attributable to disinhibition of adipose tissue lipolysis, secondary to adipose tissue insulin resistance and the absence of compensatory hyperinsulinaemia. Furthermore, hyperglycaemia in long-standing type 2 diabetes patients is associated with elevated plasma glucose appearance and disappearance rates, but is not accompanied by elevated carbohydrate oxidation rates. Moderate-intensity exercise improves plasma glucose disposal in long-standing type 2 diabetes patients, thereby effectively reducing blood glucose concentrations.
Abbreviations
- AU:
-
arbitrary unit
- IMTG:
-
intramyocellular triacylglycerol
- Pr13CO2 :
-
production of 13CO2
- Ra:
-
rate of appearance
- Rd:
-
rate of disappearance
- Rox:
-
rate of oxidation
- TTR:
-
plasma enrichment (tracer/tracee ratio)
- VO2 :
-
oxygen uptake
- VCO2 :
-
CO2 production
- VO2max :
-
maximal oxygen uptake capacity
- Wmax :
-
maximal workload capacity
References
Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL (1999) Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes 48:839–847
van Loon LJC, Koopman R, Manders R, van der Weegen W, van Kranenburg GP, Keizer HA (2004) Intramyocellular lipid content in type 2 diabetes patients compared to overweight sedentary men and highly trained endurance athletes. Am J Physiol Endocrinol Metab 287:E558–EE565
Thamer C, Machann J, Bachmann O et al (2003) Intramyocellular lipids: anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. J Clin Endocrinol Metab 88:1785–1791
Yu C, Chen Y, Cline GW et al (2002) Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277:50230–50236
Shulman GI (2000) Cellular mechanisms of insulin resistance. J Clin Invest 106:171–176
Roden M, Price TB, Perseghin G et al (1996) Mechanism of free fatty acid-induced insulin resistance. J Clin Invest 97:2859–2865
Russell AP, Gastaldi G, Bobbioni-Harsch E et al (2003) Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: a case of good vs. bad lipids? FEBS Lett 551:104–106
Adams JM 2nd, Pratipanawatr T, Berria R et al (2004) Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53:25–31
Ellis BA, Poynten A, Lowy AJ et al (2000) Long-chain acyl-CoA esters as indicators of lipid metabolism and insulin sensitivity in rat and human muscle. Am J Physiol Endocrinol Metab 279:E554–E560
Itani SI, Ruderman NB, Schmieder F, Boden G (2002) Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51:2005–2011
Blaak EE, Wagenmakers AJM, Glatz JFC et al (2000) Plasma FFA utilization and fatty acid-binding protein content are diminished in type 2 diabetic muscle. Am J Physiol Endocrinol Metab 279:E146–E154
Felber JP, Ferrannini E, Golay A et al (1987) Role of lipid oxidation in pathogenesis of insulin resistance of obesity and type II diabetes. Diabetes 36:1341–1350
Golay A, Felber JP, Meyer HU, Curchod B, Maeder E, Jequier E (1984) Study on lipid metabolism in obesity diabetes. Metabolism 33:111–116
Kelley DE, Simoneau J-A (1994) Impaired free fatty acid utilization by skeletal muscle in non-insulin dependent diabetes mellitus. J Clin Invest 94:2349–2356
Kang J, Kelley DE, Robertson RJ et al (1999) Substrate utilization and glucose turnover during exercise of varying intensities in individuals with NIDDM. Med Sci Sports Exerc 31:82–89
Blaak EE, van Aggel-Leijssen DPC, Wagenmakers AJM, Saris WHM, van Baak MA (2000) Impaired oxidation of plasma-derived fatty acids in type 2 diabetic subjects during moderate-intensity exercise. Diabetes 49:2102–2107
Borghouts LB, Wagenmakers AJM, Goyens PLL, Keizer HA (2002) Substrate utilization in non-obese type II diabetic patients at rest and during exercise. Clin Sci 103:559–566
Colberg SR, Hagberg JM, McCole SD, Zmuda JM, Thompson PD, Kelley DE (1996) Utilization of glycogen but not plasma glucose is reduced in individuals with NIDDM during mild-intensity exercise. J Appl Physiol 81:2027–2033
Taskinen MR, Bogardus C, Kennedy A, Howard BV (1985) Multiple disturbances of free fatty acid metabolism in noninsulin-dependent diabetes. Effect of oral hypoglycemic therapy. J Clin Invest 76:637–644
Kelley DE, Mandarino LJ (1990) Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus. J Clin Invest 86:1999–2007
Mensink M, Blaak EE, van Baak MA, Wagenmakers AJM, Saris WHM (2001) Plasma free fatty acid uptake and oxidation are already diminished in subjects at high risk for developing type 2 diabetes. Diabetes 50:2548–2554
van Loon LJC (2004) Use of intramuscular triacylglycerol as a substrate source during exercise in humans. J Appl Physiol 97:1170–1187
Minuk HL, Vranic M, Marliss EB, Hanna AK, Albisser AM, Zinman B (1981) Glucoregulatory and metabolic response to exercise in obese noninsulin-dependent diabetes. Am J Physiol 240:E458–E464
Martin IK, Katz A, Wahren J (1995) Splanchnic and muscle metabolism during exercise in NIDDM patients. Am J Physiol 269:E583–E590
Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15:539–553
Siri WE (1956) The gross composition of the body. Adv Biol Med Phys 4:239–280
van Loon LJC, Koopman R, Schrauwen P, Stegen J, Wagenmakers AJM (2003) The use of the [1,2-13C] acetate recovery factor in metabolic research. Eur J Appl Physiol 89:377–383
Bergstrom J (1975) Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35:609–616
Peronnet F, Massicotte D (1991) Table of nonprotein respiratory quotient: an update. Can J Sport Sci 16:23–29
Wolfe RR, Jahoor F (1990) Recovery of labeled CO2 during the infusion of C-1- vs C-2-labeled acetate: implications for tracer studies of substrate oxidation. Am J Clin Nutr 51:248–252
Sidossis LS, Coggan AR, Gastaldelli A, Wolfe RR (1995) A new correction factor for use in tracer estimations of plasma fatty acid oxidation. Am J Physiol 269:E649–E656
Schrauwen P, van Aggel-Leijssen DPC, van Marken Lichtenbelt WD, van Baak MA, Gijsen AP, Wagenmakers AJM (1998) Validation of the [1,2-13C] acetate recovery factor for correction of [U-13C] palmitate oxidation rates in humans. J Physiol 513:215–223
Jeukendrup AE, Raben A, Gijsen A et al (1999) Glucose kinetics during prolonged exercise in highly trained human subjects: effect of glucose ingestion. J Physiol 515:579–589
Trimmer JK, Casazza GA, Horning MA, Brooks GA (2001) Recovery of (13)CO2 during rest and exercise after [1-(13)C]acetate, [2-(13)C]acetate, and NaH(13)CO3 infusions. Am J Physiol Endocrinol Metab 281:E683–E692
Schaart G, Hesselink RP, Keizer HA, van Kranenburg G, Drost MR, Hesselink MK (2004) A modified PAS stain combined with immunofluorescence for quantitative analyses of glycogen in muscle sections. Histochem Cell Biol 122:161–169
Sacchetti M, Olsen DB, Saltin B, van Hall G (2005) Heterogeneity in limb fatty acid kinetics in type 2 diabetes. Diabetologia 48:938–945
Campbell PJ, Carlson MG, Nurjhan N (1994) Fat metabolism in human obesity. Am J Physiol 266:E600–E605
Eriksson JW, Smith U, Waagstein F, Wysocki M, Jansson PA (1999) Glucose turnover and adipose tissue lipolysis are insulin-resistant in healthy relatives of type 2 diabetes patients: is cellular insulin resistance a secondary phenomenon? Diabetes 48:1572–1578
Frayn KN (2005) Obesity and metabolic disease: is adipose tissue the culprit? Proc Nutr Soc 64:7–13
Groop LC, Bonadonna RC, DelPrato S et al (1989) Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 84:205–213
Martin WH (1997) Effect of endurance training on fatty acid metabolism during whole body exercise. Med Sci Sports Exerc 29:635–639
Hayashi T, Wojtaszewski JF, Goodyear LJ (1997) Exercise regulation of glucose transport in skeletal muscle. Am J Physiol 273:E1039–E1051
Blaak EE (2003) Fatty acid metabolism in obesity and type 2 diabetes mellitus. Proc Nutr Soc 62:753–760
Schrauwen P, Hesselink MKC (2004) Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes 53:1412–1417
Blaak EE, Wagenmakers AJ (2002) The fate of [U-(13)C]palmitate extracted by skeletal muscle in subjects with type 2 diabetes and control subjects. Diabetes 51:784–789
Acknowledgements
We gratefully acknowledge the expert analytical assistance of J. Stegen, J. Senden, M. Meers, H. Vandereyt and A. Gijsen, and the enthusiastic participation of the volunteers in these trials.
Duality of interest
None of the authors has a duality of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boon, H., Blaak, E.E., Saris, W.H.M. et al. Substrate source utilisation in long-term diagnosed type 2 diabetes patients at rest, and during exercise and subsequent recovery. Diabetologia 50, 103–112 (2007). https://doi.org/10.1007/s00125-006-0482-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-006-0482-2