Abstract
Aims/hypothesis
In order to test the hypothesis that disturbances in skeletal muscle fatty acid metabolism with type 2 diabetes are not equally present in the upper and lower limbs, we studied fatty acid kinetics simultaneously across the arm and leg of type 2 diabetic patients (n=6) and matched control subjects (n=7) for 5 h under baseline conditions and during a 4-h hyperinsulinaemic–euglycaemic clamp.
Methods
Limb fatty acid kinetics was determined by means of continuous [U-13C]palmitate infusion and measurement of arteriovenous differences.
Results
The systemic palmitate rate of appearance was 3.6±0.4 and 2.7±0.3 μmol·kg lean body mass−1·min−1 and decreased during the clamp by 26% (p=0.04) and 43% (p<0.01) in the diabetic patients and in the control subjects respectively. At baseline, palmitate uptake across the arm was similar in the two groups, whereas leg palmitate uptake was lower than in the arm in the diabetic patients. During the clamp, palmitate uptake decreased in the arm (−48%, p=0.02) and the leg (−38%, p=0.04) of the control subjects, whereas it decreased in the arm (−30%, p=0.04) but not in the leg of the diabetic patients. Similarly, during the clamp palmitate release was substantially suppressed in the arm (−47%, p<0.01) and the leg of the control subjects (−45%, p<0.01), but only in the arm of the diabetic patients (−45%, p<0.01).
Conclusions/interpretation
The present data indicate that type 2 diabetes is characterised by heterogeneity in the dysregulation of skeletal muscle fatty acid metabolism, with only the leg, but not the arm, showing an impairment of fatty acid kinetics at baseline and during a hyperinsulinaemic–euglycaemic clamp causing a physiological increase in insulin concentration.
Similar content being viewed by others
Introduction
Disturbances of fatty acid metabolism may play a role in the development of insulin resistance and type 2 diabetes. In the postabsorptive state type 2 diabetic patients have an increased plasma fatty acid concentration [1, 2], which may interfere with glucose homeostasis/metabolism. In healthy volunteers it has been shown that an elevation of plasma fatty acid levels for some hours induced insulin resistance [3–5] and that a reduction in fatty acid level reduced the severity of insulin resistance in type 2 diabetic patients [6].
Skeletal muscle is an important tissue for the maintenance of glucose and fatty acid homeostasis in the body, and being a prime target for insulin action is therefore linked to insulin resistance. Reduced plasma fatty acid uptake and oxidation by the leg [7] and the arm [8, 9] have been observed in type 2 diabetics as compared to healthy individuals. Moreover, the relationship between intramuscular lipid accumulation and insulin resistance has been described [10, 11]. Physical inactivity or muscle disuse is recognised as one of the risk factors for the development of insulin resistance and type 2 diabetes [12, 13]. Against this, it has been shown that exercise training can reduce insulin resistance in the leg of diabetic patients [14, 15]. Recently, evidence has been presented for heterogeneity towards lipolysis in human skeletal muscles [16]. Given the different roles of arm and leg muscles and their different activation in daily life, it is possible that the observed disturbances in skeletal muscle fatty acid kinetics in type 2 diabetes are not equal in skeletal muscles of the arms and legs. To test this hypothesis, we investigated limb fatty acid kinetics by means of a combination of stable isotope dilution and an arterial–venous balance technique applied to the arm and the leg of sedentary type 2 diabetic patients and matched healthy volunteers under baseline conditions and during a hyperinsulinaemic–euglycaemic clamp causing an increase in insulin well within the physiological range.
Subjects, materials and methods
Subjects
Six type 2 diabetic male volunteers and seven matched healthy volunteers (controls) participated in the study. The characteristics of the subjects are reported in Table 1. All subjects had had a stable body weight for at least the preceding 3 months and were not engaged in any regular strenuous physical activity or have a physically demanding job. Two of the diabetic patients were treated with diet only, one patient with diet and insulin, and three patients with diet and oral glucose-lowering agents (two patients with metformin and one patient with metformin and sulphonylureas). All medications were withheld 24 h before the experiments. None of the patients had health problems, apart from type 2 diabetes. None of the healthy control subjects were receiving medication and all had a normal oral glucose tolerance test. The patients and healthy volunteers were informed about the aim and the possible risks involved in the study and gave their written consent to participation. The study was conducted according to the Declaration of Helsinki and had been approved by the Ethical Committee of Copenhagen–Frederiksberg Communities.
Body composition assessment
Body composition was determined by dual-energy X-ray absorptiometry, which has been shown to provide a valid and reproducible estimation of limb fat and muscle mass [17]. Before each scan the machine was calibrated with phantoms of known composition.
Experimental procedure
The type 2 diabetic patients and control subjects refrained from any of their ordinary physical activities during the 48 h preceding the trial. On the day of the experiment the subjects reported to the laboratory at 08.00 hours after an overnight fast (12 h). After 20 min of supine rest, Teflon catheters (20 G; Ohmeda, Wiltshire, UK) were inserted in the femoral artery and in the retrograde direction into the femoral vein of one leg using the Seldinger technique. In addition, a 16 G catheter was inserted into an antecubital vein of one arm and the tip of the catheter advanced into the subclavian vein until approximately 5 cm before the merger with the jugular vein. The positioning of the tip of the catheter was verified by X-ray. Furthermore, two catheters were placed in antecubital veins of the other arm for stable isotope, glucose and insulin infusion. Twenty minutes after the catheterisation procedure, baseline blood samples were taken and femoral and subclavian blood flow was measured by Doppler ultrasound [18]. Subsequently, a constant infusion of [U-13C]palmitate (0.009 μmol·kg−1·min−1; Cambridge Isotope Laboratories, Andover, MA, USA) was started. The isotope infusion was continued for 9 h, during which time the subjects remained in the supine position. Blood sampling and blood flow measurements were performed every 60 min throughout the experiment. Before blood sampling, a pneumatic cuff was placed under the knee and around the wrist and inflated to a suprasystolic pressure to avoid shunting in the lower leg and in the hand [19]. Five hours after the start of the stable isotope infusion, an insulin infusion (1 pmol kg−1·min−1) was started and continued for the remaining 4 h of the study. During the hyperinsulinaemic clamp the arterial glucose concentration was maintained at the value recorded at the end of the baseline period (5.07±0.1 mmol/l in the control subjects and 6.86±1.0 mmol/l in the diabetic patients) by infusion of a 20% glucose solution. The [U-13C]palmitate infusion rate was reduced by 50% at the start of the hyperinsulinaemic–euglycaemic clamp. Arterial glucose and potassium concentrations were measured every 10 min during the clamp period (ABL 715; Radiometer, Copenhagen, Denmark) and potassium was infused to maintain the plasma potassium concentration.
Analytical procedures
Blood for the measurement of substrate concentrations and isotopic enrichment was collected in prechilled tubes containing 0.3 M EDTA (10 μl ml−1 blood) and immediately centrifuged for 10 min at 4°C. The plasma obtained was immediately frozen and stored at −80°C until analysis. Plasma insulin was determined with an ELISA kit (Dako, Glostrup, Denmark). Extraction of plasma fatty acid for the determination of palmitate concentration and isotopic enrichment was performed according to Patterson and colleagues [20]. Palmitate concentration was measured by gas chromatography with FID (Autosystem XL; Perkin Elmer, Northwalk, CT, USA), using heptadecanoic acid as an internal standard. Plasma palmitate 13C enrichment was measured by gas chromatography-combustion isotope ratio mass spectrometry (GC-C-IRMS, Hewlett Packard 5890, Finnigan GC combustion III, Finnigan Deltaplus; Finnigan MAT, Bremen, Germany) as described previously [21]. Palmitate enrichment was corrected by a factor of 17/16 to account for the extra methyl group of the methyl palmitate derivative.
Calculations
Whole-body palmitate kinetics
The whole-body rate of appearance (R a) of palmitate in plasma, which in steady-state conditions is equal to the rate of disappearance (R d), was calculated by dividing the tracer infusion rate by the arterial plasma enrichment (Steele’s equation for steady-state conditions [22]).
Limb substrate balance and kinetics
Net palmitate balance across the leg and the arm was calculated by multiplying the arterial–venous concentration difference by plasma flow, calculated as blood flow×(1−haematocrit). The limb fractional extraction was calculated as
where C a and E a, and C v and E v are the concentration and the tracer enrichment of palmitate in the artery and the femoral/subclavian vein respectively. The limb palmitate uptake was calculated as
and the limb release as
Statistical analysis
Data are expressed as means±SE. The data are presented as average values of the 2–5 h (Basal) and 6–9 h (Insulin) periods, when an apparent isotopic and physiological steady state was achieved. Differences between the average values of the two periods, groups and limbs were determined by two-way repeated-measures analysis of variance (ANOVA). When ANOVA revealed a significant effect, a t-test was used to determine where the difference occurred. When the comparison between limbs was not applicable, differences between groups were determined with the unpaired t-test, whereas the effect of the clamp was determined with the paired t-test. Statistical significance was accepted at a p value of less than 0.05.
Results
Arterial and systemic palmitate rate of appearance (R a)
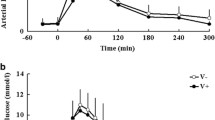
The arterial palmitate concentration was similar in diabetic patients and control subjects during the last 3 h of the baseline and hyperinsulinaemic–euglycaemic clamp period and was substantially decreased during the clamp (Fig. 1). Baseline palmitate R a was 3.6±0.4 and 2.7±0.3 μmol·kg lean body mass−1·min−1 in the diabetic patients and in the control subjects respectively (Fig. 1). Palmitate R a decreased substantially during the hyperinsulinaemic–euglycaemic clamp in the control subjects (−43%, p<0.01) and the diabetic patients (−26%, p<0.05).
Arterial palmitate and systemic palmitate rate of appearance (R a) in control subjects and type 2 diabetic patients. a Arterial palmitate concentration and b systemic palmitate R a in control subjects and in type 2 diabetic patients during the baseline (basal, filled bars) and hyperinsulinaemic–euglycaemic clamp (insulin, open bars) periods. *p<0.05 for difference from basal
Limb palmitate kinetics
Under baseline conditions a net palmitate uptake was observed across the leg, whereas a net palmitate release was observed across the arm in both groups (Fig. 2). The unidirectional palmitate uptake was normalised for the limb muscle mass and palmitate release for the limb fat mass in order to compare arm and leg kinetics. In control subjects, palmitate uptake was not significantly different between the limbs during the baseline period and decreased similarly during the clamp (−48% and −38% for the arm and the leg, respectively). However, in the diabetic patients palmitate uptake by the leg was lower than that by the arm (p<0.05) (Fig. 3). During the hyperinsulinaemic–euglycaemic clamp, palmitate uptake was substantially decreased in the arm of the diabetic patients compared with baseline (−30%, p=0.02), whereas it was unchanged in the leg. Leg and arm blood flow was not significantly influenced by insulin infusion (Table 2).
Unidirectional limb palmitate uptake and release in control subjects and type 2 diabetic patients. a Limb unidirectional palmitate uptake (expressed per kg muscle mass) and b release (expressed per kg fat mass) during the baseline (basal, filled bars) and hyperinsulinaemic–euglycaemic clamp (insulin, open bars) periods. * p<0.05 for significant difference from basal; # p<0.05 for significant difference from leg
The unidirectional palmitate release is a dependent variable of the tracer-determined palmitate uptake and the net palmitate balance across the limbs. Palmitate release was higher in the arm than in the leg under baseline conditions and during the clamp in control and diabetic subjects (Fig. 3). During the hyperinsulinaemic–euglycaemic clamp a substantial decrease in arm palmitate release was observed in both groups. In contrast, and similarly to what was observed for palmitate uptake, during the clamp palmitate release was unchanged in the leg of the diabetic patients but substantially suppressed in the leg of the control subjects (−47%, p<0.01).
Contribution of limb palmitate uptake/release to systemic palmitate R d/R a
Under baseline conditions the fractional contribution of palmitate uptake by the arm to systemic palmitate R d was similar in control subjects and diabetic patients, whereas the contribution of the leg was substantially lower in diabetic patients during the baseline and clamp period (Fig. 4).
Limb fractional contribution to systemic palmitate turnover in healthy control subjects and type 2 diabetic patients. Limb fractional contribution to systemic palmitate R d (a) and R a (b) in control subjects and type 2 diabetic patients during the baseline (basal, filled bars) and hyperinsulinaemic–euglycaemic clamp (insulin, open bars) periods. † p<0.05 for significant difference from control
Insulin concentration and plasma palmitate enrichment
Arterial plasma insulin concentration during the last 3 h of the baseline period averaged 39±12 and 68±14 pmol/l in the control subjects and the diabetic patients, respectively. During the hyperinsulinaemic–euglycaemic clamp the insulin concentration increased to 81±11 and 141±24 pmol/l in the control subjects and the diabetic patients, respectively. The reduction of the [U-13C]palmitate infusion rate at the beginning of the clamp period successfully minimised the changes in palmitate enrichment expected from the effect of insulin infusion (Fig. 5).
Discussion
The present study investigated arm and leg fatty acid kinetics in postabsorptive healthy volunteers and diabetic patients under baseline conditions and during a hyperinsulinaemic–euglycaemic clamp. The main findings were that: (1) palmitate uptake is similar in arm and leg of healthy individuals under baseline conditions and during a hyperinsulinaemic–euglycaemic clamp; (2) palmitate release, indicative of lipolysis, is substantially higher in the arm than in the leg; and (3) during a hyperinsulinaemic–euglycaemic clamp fatty acid kinetics is similar in the arm but markedly different in the leg in diabetic patients compared with healthy individuals. Thus, our hypothesis that disturbances in skeletal muscle fatty acid kinetics in type 2 diabetes are not equal in arm and leg muscles was confirmed.
In the present study, fatty acid kinetics was simultaneously determined for the arm and leg in order to determine potential differences in limb fatty acid metabolism. Because of the importance of fully understanding the dysregulation of fatty acid metabolism in type 2 diabetes, we also determined the effect of insulin on limb fatty acid kinetics using a hyperinsulinaemic–euglycaemic clamp in healthy individuals and diabetic patients. Since insulin is one of the most potent antilipolytic hormones [23], a low dose of insulin was infused with the intention of inducing an increase in insulin levels well within the normal physiological range and to prevent the complete blockage of lipolysis. Type 2 diabetes has been shown to be associated with impaired suppression of fatty acid levels and fatty acid R a by insulin [24, 25]. Accordingly, in the present study a tendency was observed for a less significant suppression of arterial palmitate concentration and systemic palmitate turnover rate in diabetic patients during the hyperinsulinaemic–euglycaemic clamp. This observation is also in agreement with the impaired postprandial suppression of systemic fatty acid turnover demonstrated in type 2 diabetic patients [26]. From the present study it is clear that the skeletal muscles of the leg more than those of the arm may play an important role in the reduced insulin sensitivity with regard to the systemic fatty acid turnover in type 2 diabetes, since no suppression was observed in palmitate uptake or release by the leg.
Palmitate uptake under baseline conditions, expressed per kilogram of muscle mass, was not significantly different between the upper and lower limbs in the control subjects, whereas it was lower in the leg of the diabetic patients. Reduced fatty acid uptake has been observed in the forearm [8] and in the leg [7, 27] of type 2 diabetic patients. A lower capacity for fatty acid uptake in the diabetic leg muscles is also in agreement with similar findings in human cultured vastus lateralis muscle cells [28]. However, subsequent data on human cultured myotubes from the vastus lateralis did not indicate substantial differences between myotubes from diabetic patients and matched controls [29]. The decrease of palmitate uptake with insulin has been reported previously for the leg [7, 30] and the arm [31] of healthy subjects. Nevertheless, when fatty acid levels were maintained during the insulin infusion, the fatty acid uptake by skeletal muscle was actually increased in rat muscle preparations [32, 33] and in cultured cells from the vastus lateralis muscle [28, 29]. These observations imply that the reduction in limb fatty acid uptake with insulin is caused more by reduced limb fatty acid availability than by lessened skeletal muscle insulin sensitivity. However, the present study clearly shows that, in spite of the markedly reduced palmitate availability, the palmitate uptake in the leg is maintained during insulin infusion in diabetic patients. This suggests an important role of insulin in the regulation of skeletal muscle fatty acid metabolism and a derangement of this regulation in type 2 diabetes, which is potentially responsible for the chronically elevated fatty acid levels. Fatty acid uptake in skeletal muscle is thought to occur, at least in part, as a protein-mediated process [34, 35]. In humans, plasma membrane fatty acid binding protein in the vastus lateralis muscle has been reported to be either diminished [8] or increased [36] in type 2 diabetes, whereas fatty acid translocase (FAT)/CD36 has been suggested to be unaltered [36]. To our knowledge, however, data on fatty acid transport proteins in arm muscles in humans are lacking. Moreover, in rat skeletal muscle, insulin has been proved to translocate FAT/CD36 from the intracellular pool to the plasma membrane, causing increased fatty acid uptake. Interestingly, protein-mediated fatty acid transport in human cultured muscle cells from the vastus lateralis has been shown to be stimulated by insulin in non-diabetic but not in type 2 diabetic subjects [28]. In the light of the results of the present study, it would be of interest to investigate the possibility of a different effect of type 2 diabetes on protein-mediated fatty acid transport in arm and leg muscles under both basal and insulin-stimulated conditions.
The limbs clearly consist not solely of skeletal muscle but also of subcutaneous adipose tissue, adipose tissue interspersed within and around the muscle bundles, and lipid droplets within the myocytes. While it has been suggested that adipose tissue has a very limited contribution to palmitate uptake in volunteers in the postabsorptive state [21, 37, 38], this is not the case for palmitate release. Furthermore, heterogeneity in adipose tissue [39] and skeletal muscle [16] lipolysis has been reported. In addition, skeletal muscle and adipose tissue lipolysis can respond differently to insulin [40–43], also in relation to the insulin level [44, 45]. Taken together, these factors hamper a clear interpretation of palmitate release in the arm and leg of healthy individuals and diabetic patients. Nevertheless, in the present study the similarity between the groups in the fat contents of the arm and leg reinforces the validity of the observation of a disturbed response of palmitate release in the leg to insulin in diabetic patients. In fact, whatever the prevalent source of palmitate release, this was suppressed differently by insulin in diabetic patients, being reduced in the arm but not in the leg.
Physical inactivity or muscle disuse is linked to the development of insulin resistance and type 2 diabetes [12, 13], whereas exercise training can reduce insulin resistance [14, 15]. The present findings of a marked difference in limb palmitate kinetics between arm and leg might be interpreted from the perspective of a different activity pattern between the two extremities. It could be argued that in sedentary individuals and with ageing, the leg undergoes a relatively more pronounced decline in muscular activity due to reduced locomotion, whereas the arm maintains better its activity level because of greater involvement in the activities of daily living, thereby being less prone to the development of insulin resistance. Whether or not this speculative explanation is true, and in view of their large muscle content, it is clear that the legs should be a prime target in the strategy to prevent insulin resistance and development of type 2 diabetes.
In conclusion, our study indicates that there is heterogeneity in the dysregulation of skeletal muscle fatty acid metabolism in type 2 diabetes in terms of fatty acid kinetics and its sensitivity to insulin stimulation, which are different in the leg but similar in the arm of type 2 diabetic patients compared with non-diabetic matched control subjects. The present data warrant future studies to elucidate the mechanism of the different metabolic perturbation in the arm and leg muscles of patients with type 2 diabetes.
Abbreviations
- FAT:
-
fatty acid translocase
- Ra :
-
rate of appearance
- Rd :
-
rate of disappearance
- [U-13C]palmitate:
-
13C uniformly labelled palmitate
References
Skowronski R, Hollenbeck CB, Varasteh BB, Chen YD, Reaven GM (1991) Regulation of non-esterified fatty acid and glycerol concentration by insulin in normal individuals and patients with type 2 diabetes. Diabet Med 8:330–333
Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD (1988) Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes 37:1020–1024
Boden G, Jadali F, White J et al (1991) Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Invest 88:960–966
Boden G (1997) Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46:3–10
Kelley DE, Mokan M, Simoneau JA, Mandarino LJ (1993) Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest 92:91–98
Santomauro AT, Boden G, Silva V et al (1999) Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 48:1836–1841
Kelley DE, Simoneau JA (1994) Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest 94:2349–2356
Blaak EE, Wagenmakers AJ, Glatz JF et al (2000) Plasma FFA utilization and fatty acid-binding protein content are diminished in type 2 diabetic muscle. Am J Physiol Endocrinol Metab 279:E146–E154
Blaak EE, Wagenmakers AJ (2002) The fate of [U-(13)C]palmitate extracted by skeletal muscle in subjects with type 2 diabetes and control subjects. Diabetes 51:784–789
Boden G, Shulman GI (2002) Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 32 [Suppl 3]:14–23
Kelley DE, Goodpaster BH (2001) Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care 24:933–941
Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS Jr (1991) Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med 325:147–152
Manson JE, Rimm EB, Stampfer MJ (1991) Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 338:774–778
Dela F, Larsen JJ, Mikines KJ, Ploug T, Petersen LN, Galbo H (1995) Insulin-stimulated muscle glucose clearance in patients with NIDDM. Effects of one-legged physical training. Diabetes 44:1010–1020
Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JF, Dela F (2004) Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes 53:294–305
Hagstrom-Toft E, Qvisth V, Nennesmo I et al (2002) Marked heterogeneity of human skeletal muscle lipolysis at rest. Diabetes 51:3376–3383
Fuller NJ, Laskey MA, Elia M (1992) Assessment of the composition of major body regions by dual-energy X-ray absorptiometry (DEXA), with special reference to limb muscle mass. Clin Physiol 12:253–266
Radegran G (1997) Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol 83:1383–1388
van Hall G, Gonzalez-Alonso J, Sacchetti M, Saltin B (1999) Skeletal muscle substrate metabolism during exercise: methodological considerations. Proc Nutr Soc 58:899–912
Patterson BW, Zhao G, Elias N, Hachey DL, Klein S (1999) Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res 40:2118–2124
Van Hall G, Bulow J, Sacchetti M, Al Mulla N, Lyngso D, Simonsen L (2002) Regional fat metabolism in human splanchnic and adipose tissues; the effect of exercise. J Physiol 543:1033–1046
Steele R (1959) Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci 82:420–430
Holm C (2003) Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans 31:1120–1124
Basu A, Basu R, Shah P, Vella A, Rizza RA, Jensen MD (2001) Systemic and regional free fatty acid metabolism in type 2 diabetes. Am J Physiol Endocrinol Metab 280:E1000–E1006
Groop LC, Bonadonna RC, DelPrato S et al (1989) Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 84:205–213
Miles JM, Wooldridge D, Grellner WJ et al (2003) Nocturnal and postprandial free fatty acid kinetics in normal and type 2 diabetic subjects: effects of insulin sensitization therapy. Diabetes 52:675–681
Kelley DE, Reilly JP, Veneman T, Mandarino LJ (1990) Effects of insulin on skeletal muscle glucose storage, oxidation, and glycolysis in humans. Am J Physiol 258:E923–E929
Wilmsen HM, Ciaraldi TP, Carter L, Reehman N, Mudaliar SR, Henry RR (2003) Thiazolidinediones upregulate impaired fatty acid uptake in skeletal muscle of type 2 diabetic subjects. Am J Physiol Endocrinol Metab 285:E354–E362
Gaster M, Rustan AC, Aas V, Beck-Nielsen H (2004) Reduced lipid oxidation in skeletal muscle from type 2 diabetic subjects may be of genetic origin: evidence from cultured myotubes. Diabetes 53:542–548
Meek SE, Nair KS, Jensen MD (1999) Insulin regulation of regional free fatty acid metabolism. Diabetes 48:10–14
Capaldo B, Napoli R, Di Marino L, Picardi A, Riccardi G, Sacca L (1988) Quantitation of forearm glucose and free fatty acid (FFA) disposal in normal subjects and type II diabetic patients: evidence against an essential role for FFA in the pathogenesis of insulin resistance. J Clin Endocrinol Metab 67:893–898
Dyck DJ, Steinberg G, Bonen A (2001) Insulin increases FA uptake and esterification but reduces lipid utilization in isolated contracting muscle. Am J Physiol Endocrinol Metab 281:E600–E607
Luiken JJ, Koonen DP, Willems J et al (2002) Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes 51:3113–3119
Bonen A, Luiken JJ, Glatz JF (2002) Regulation of fatty acid transport and membrane transporters in health and disease. Mol Cell Biochem 239:181–192
Hajri T, Abumrad NA (2002) Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annu Rev Nutr 22:383–415
Bruce CR, Anderson MJ, Carey AL et al (2003) Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J Clin Endocrinol Metab 88:5444–5451
van Hall G, Sacchetti M, Radegran G, Saltin B (2002) Human skeletal muscle fatty acid and glycerol metabolism during rest, exercise and recovery. J Physiol 543:1047–1058
Coppack SW, Persson M, Judd RL, Miles JM (1999) Glycerol and nonesterified fatty acid metabolism in human muscle and adipose tissue in vivo. Am J Physiol 276:E233–E240
Jensen MD (1997) Lipolysis: contribution from regional fat. Annu Rev Nutr 17:127–139
Bolinder J, Kerckhoffs DA, Moberg E, Hagstrom-Toft E, Arner P (2000) Rates of skeletal muscle and adipose tissue glycerol release in nonobese and obese subjects. Diabetes 49:797–802
Hagstrom-Toft E, Thorne A, Reynisdottir S et al (2001) Evidence for a major role of skeletal muscle lipolysis in the regulation of lipid oxidation during caloric restriction in vivo. Diabetes 50:1604–1611
Sjostrand M, Gudbjornsdottir S, Holmang A, Strindberg L, Ekberg K, Lonnroth P (2002) Measurements of interstitial muscle glycerol in normal and insulin-resistant subjects. J Clin Endocrinol Metab 87:2206–2211
Navegantes LC, Sjostrand M, Gudbjornsdottir S, Strindberg L, Elam M, Lonnroth P (2003) Regulation and counterregulation of lipolysis in vivo: different roles of sympathetic activation and insulin. J Clin Endocrinol Metab 88:5515–5520
Jacob S, Hauer B, Becker R et al (1999) Lipolysis in skeletal muscle is rapidly regulated by low physiological doses of insulin. Diabetologia 42:1171–1174
Stumvoll M, Jacob S, Wahl HG et al (2000) Suppression of systemic, intramuscular, and subcutaneous adipose tissue lipolysis by insulin in humans. J Clin Endocrinol Metab 85:3740–3745
Acknowledgements
The study was supported by The Danish National Research Foundation (grant 504-14). Massimo Sacchetti was a recipient of a research grant from Istituto Universitario di Scienze Motorie, Rome, Italy. We thank Nina Pluszek, Heidi Hansen and Carsten Nielsen for valuable technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sacchetti, M., Olsen, D.B., Saltin, B. et al. Heterogeneity in limb fatty acid kinetics in type 2 diabetes. Diabetologia 48, 938–945 (2005). https://doi.org/10.1007/s00125-005-1727-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-1727-1