Abstract
Aims/hypothesis
Impaired nitric oxide (NO) bioactivity and increased superoxide (SO) production are characteristics of vascular endothelial dysfunction in diabetes. The underlying mechanisms remain unknown. In this regard, we investigated the role of tetrahydrobiopterin (BH4) bioavailability in regulating endothelial nitric oxide synthase (eNOS) activity, dimerisation and SO production in streptozotocin-induced diabetic mice.
Methods
Mouse aortas were used for assays of the following: (1) aortic function by isometric tension; (2) NO by electronic paramagnetic resonance; (3) SO by lucigenin-enhanced chemiluminescence and dihydroethidine fluorescence; (4) total biopterin and BH4 by high-performance liquid chromatography; and (5) eNOS protein expression and dimerisation by immunoblotting.
Results
In diabetic mouse aortas, relaxations to acetylcholine and NO levels were significantly decreased, but SO production was increased, in association with reductions in total biopterins and BH4. Although total eNOS levels were increased in diabetes, the protein mainly existed in monomeric form. Conversely, specifically augmented BH4 in diabetic endothelium preserved eNOS dimerisation, but the expression remained unchanged.
Conclusions/interpretation
Our results demonstrate that BH4 plays an important role in regulating eNOS activity and its functional protein structure, suggesting that increasing endothelial BH4 and/or protecting it from oxidation may be a rational therapeutic strategy to restore eNOS function in diabetes.
Similar content being viewed by others
Introduction
Diabetic patients are at greatly increased risk of cardiovascular, cerebrovascular and peripheral vascular disease, leading to myocardial infarction, strokes and amputations. Endothelial dysfunction is an early pathophysiological feature of diabetic vascular disease, characterised by a decrease in nitric oxide (NO) bioavailability and a concomitant increase in vascular superoxide (SO) formation in endothelium [1]. Loss of endothelial NO synthase (eNOS)-derived NO bioavailability in diabetic vascular disease results in vasospasm, platelet aggregation, leucocyte adhesion and vascular smooth muscle proliferation [1, 2]. Simultaneously, the increased SO production inhibits eNOS NO biosynthesis [3, 4] and degrades NO [5], thus further contributing to diabetic endothelial dysfunction. Under these conditions, eNOS ‘uncoupling’ results in SO formation over NO production [6–8].
Tetrahydrobiopterin (BH4) is required for all NOS bioactivity [8, 9]. BH4 de novo biosynthesis is regulated by the rate-limiting enzyme, GTP cyclohydrolase I (GTPCH; EC 3.5.4.16), which converts GTP to dihydroneopterin triphosphate. BH4 is finally produced through further steps catalysed by 6-pyruvol-tetrahydropterin synthase and sepiapterin reductase [10]. BH4 appears to facilitate electron transfer from the reductase domain to the oxygenase domain of NOS, and maintains the haem prosthetic group in its redox active form [6, 7, 11]. Furthermore, BH4 also promotes and possibly stabilises eNOS protein in the active homodimer formation [12–16].
Loss of BH4 is a feature of diabetes, possibly owing to increased oxidative stress [1]. A major active oxidant, peroxynitrite, formed by NO and SO interaction, is capable of rapidly oxidising BH4 [5], which, in turn, can lead to eNOS dysfunction [9, 17]. Acute and chronic supplementation of BH4 in experimental models appears to improve endothelial function [4, 18–20]. Similarly, in patients with diabetes, a beneficial effect of BH4 supplementation on forearm blood flow was observed [21]. However, the mechanistic links between BH4 deficiency, eNOS activity and dimerisation in diabetes remain unclear.
In the present study, we investigated the changes in BH4 levels and their association with eNOS function, particularly protein dimerisation, in the aortas of streptozotocin (STZ)-induced diabetic mice. We also investigated the role of BH4 in eNOS dimerisation in diabetes using the GTPCH transgenic (GCH-Tg) mouse, in which BH4 biosynthesis is specifically augmented in the vascular endothelium [22].
Materials and methods
Animals and induction of diabetes by streptozotocin injection
The mice used in this study were 12- to 14-week-old males and included the strains Balb/c (Harlan Sprague–Dawley, Bicester, UK), C57BL/6 (Harlan Sprague–Dawley) and GCH-Tg (specific overexpression of GTPCH in a C57BL/6 background, as previously described) as well as their wild-type littermates [22]. The experiments were conducted in accordance with the UK Home Office Animals (Scientific Procedures) Act of 1986.
We have previously shown that STZ-induced diabetes in C57BL/6 mice results in endothelial dysfunction [22]. To ensure that our findings were not specific to the C57BL/6 strain, we carried out similar experiments in Balb/c mice. Diabetes was induced by peritoneal injection of STZ (Sigma-Aldrich, Gillingham, UK), 200 mg/kg for Balb/c and 160 mg/kg for C57BL/6 and GCH-Tg, or of STZ vehicle (citrate buffer; control), as previously described [22–24]. Blood glucose was monitored weekly using a one-touch blood glucose meter (Lifescan, High Wycombe, UK); body weight was also measured weekly. Four weeks after STZ injection, blood glucose levels increased from 6.94±0.33 to 29.47±1.68 mmol/l (means±SEM) for Balb/c and from 7.5±0.43 to 31.95±0.82 mmol/l for C57BL/6, in association with weight loss from 29.8±0.51 to 24.9±0.5 g for Balb/c and from 28±0.76 to 23±0.63 g for C57BL/6. At this time point, mice were killed and perfused with 2 to 5 ml of cold PBS, and the thoracic aortas were harvested and prepared for experiments.
Isometric tension vasomotor studies
Aortic vasomotor function was studied using organ bath chambers (Multi-Myograph 610M; Danish Myo Technology, Aarhus, Denmark), as previously described [22]. Briefly, two rings, each 2 mm in length, were divided from each thoracic aorta. Vessel rings were constricted with 60 mmol/l KCl, typically achieving a contraction of 5 to 7 mN of active tension after 5 min. Vessel rings contraction responses to cumulative concentration (10−9 to 10−5 mol/l) of phenylephrine were expressed as a percentage of the maximal KCl response. Vessels were washed three times with fresh Krebs-HEPES buffer, equilibrated for 30 min, and then precontracted to approximately 90% of maximal tension with phenylephrine (typically 3×10−6 mol/l). Relaxation to cumulative concentration of acetylcholine (10−9 to 10−5 mol/l) was expressed as a percentage of the phenylephrine preconstruction. After three washes, vessels were precontracted again with phenylephrine, and 10−4 mol/l\(N^{\omega } \)-nitro-l-arginine methyl ester (Sigma-Aldrich) was added to inhibit endogenous NOS activity. Finally, sodium nitroprusside (10−10 to 10−6 mol/l) was added to evaluate endothelium-independent relaxation, expressed as a percentage of the phenylephrine precontraction.
NO production assays by electron paramagnetic resonance
NO production in the aorta was assayed using colloid Fe-diethyldithiocarbamate (DETC)2 spin-trapping and electron paramagnetic resonance (EPR) spectroscopy [25]. Colloid Fe(DETC)2 was made by separately dissolving 3.6 mg NaDETC (Alexis, Maidenhead, UK) and 2.25 mg FeSO4·7H2O (BDH Laboratory Supplies, Poole, UK) in 10 ml ice-cold Krebs-HEPES buffer (NaCl 99 mmol/l, KCl 4.7 mmol/l, MgSO4 1.2 mmol/l, KH2PO4 1.0 mmol/l, CaCl2 1.9 mmol/l, NaHCO3 25 mmol/l, HEPES 20 mmol/l) under argon gas bubbling, and was mixed immediately before use. Each vessel was longitudinally opened and incubated for 90 min in 350 μl Krebs-HEPES buffer containing 0.4 mmol/l colloid Fe(DETC)2, calcium ionophore (A23187; 1 μmol/l) and l-arginine (100 μmol/l). Vessels were then frozen using liquid nitrogen, and EPR spectra were obtained using an X-band EPR spectrometer (Miniscope MS 200; Magnettech, Berlin, Germany). Instrument settings were as follows: centre-field (Bo) 3276G, sweep 115G, microwave power 10 mW, modulation frequency 100 kHz, amplitude modulation 8,000 mG, sweep time 60 s, and number of scans 4. Total amplitude and the NO-Fe(DETC)2 signal were measured after correction of baseline and expressed as arbitrary units/mg dry weight of vessel.
SO production assays
Two different methods were used to measure SO production in aortas, as previously described [22]: lucigenin-enhanced chemiluminescence and dihydroethidine fluorescence. SO in the mouse aorta was measured using 10 μmol/l lucigenin-enhanced chemiluminescence. The whole thoracic aorta was harvested, flushed with Krebs-HEPES buffer, and opened longitudinally. The aorta was gassed with 95% O2/5% CO2 in warmed Krebs-HEPES buffer for 30 min before measurement of chemiluminescence using an FB12 luminometer (Berthold Detection Systems, Pforzheim, Germany). A baseline reading was obtained for 4 min before the vessel was added. Then the vessel was allowed to equilibrate and adapt to the dark for 5 min before mean chemiluminescence was recorded for 10 min. Results were expressed as counts per second per mg of vessel dry weight. SO production in tissue sections of aorta was also detected using dihydroethidine fluorescence. A segment of mouse aorta was frozen in optimum cutting temperature compound and sectioned (30 μm). After incubation (in the dark at 37°C for 10 min) with Krebs-HEPES buffer containing 2 μmol/l dihydroethidine (Molecular Probes, Leiden, The Netherlands), in the presence or absence of 500 U/ml polyethylene-glycol-conjugated superoxide dismutase (Sigma-Aldrich), the sections were washed immediately with ice-cold Krebs-HEPES buffer and imaged using a Bio-Rad MRC1024 confocal microscope (Hemel Hempstead, UK).
Measurement of biopterins in mouse aortas
Measurement of biopterin levels in aortic lysates was carried out as described previously [15, 22]. Briefly, the fresh thoracic aorta was homogenised in cold extract buffer (50 mmol/l Tris–HCl, pH 7.4, 1 mmol/l dithiothreitol, 1 mmol/l EDTA). Protein concentration in supernatant was measured using the Bio-Rad protein assay. Proteins were removed by adding 10 μl of a 1:1 mixture of 1.5 mol/l HClO4 and 2 mol/l H3PO4 to 90 μl of extracts, followed by centrifugation. To measure total biopterins (BH4, BH2, biopterin) by acid oxidation, 10 μl of 1% iodine in 2% KI solution was added to 90 μl protein-free supernatant. To measure BH2 and biopterin by alkali oxidation, 10 μl of 1 mol/l NaOH was added to 80 μl of extract, then the same was done with 10 μl of iodine/KI solution. Samples were incubated at room temperature for 1 h in the dark. Alkaline oxidation samples were then acidified with 20 μl of 1 mol/l H3PO4. Iodine was reduced by adding 5 μl of fresh ascorbic acid (20 mg/ml). HPLC was performed using an Ace 5 C18 column (Advanced Chromatography Technologies, Aberdeen, UK) with a 10% methanol/90% water mobile phase run at 0.4 ml/min. Fluorescence detection (350 nm excitation, 450 nm emission) was performed using an FP-2020 detector (Jasco, Great Dunmow, UK). BH4 concentration, expressed as pmol/mg protein, was calculated by subtracting BH2 plus biopterin from total biopterins.
Western blot analysis
Western blot analysis was performed as previously described [15]. Briefly, the whole vessel was opened longitudinally and lysed for 1 h at 4°C in 40 μl of lysis buffer (50 mmol/l Tris–HCl pH 8, 0.2% Nonidet P-40, 180 mmol/l NaCl, 0.5 mmol/l EDTA, 100 mmol/l phenylmethylsulphonyl fluoride, 1 mol/l dithiothreitol, and protease inhibitors). An equal amount of cellular protein was resolved by 6% SDS-PAGE and transferred to polyvinylidine difluoride membranes. To investigate eNOS homodimer formation in the aortic endothelial cells, a non-boiled sample was resolved by 6% SDS-PAGE at 4°C. Membranes were incubated with a 1:2,000 dilution of mouse anti-eNOS monoclonal antibody (BD Transduction Laboratories, Oxford, UK) or a 1:1,000 dilution of rat anti-haemaglutinin high-affinity monoclonal antibody [26]. Bands were visualised using chemiluminescence, and quantified using National Institutes of Health image software.
Statistics
Results are expressed as means±SEM. Statistical significance of differences between means was assessed using Student’s unpaired two-tailed t-test. A p value of less than 0.05 was considered statistically significant.
Results
Endothelial-dependent relaxation and NO bioavailability in the diabetic aorta of the Balb/c mouse
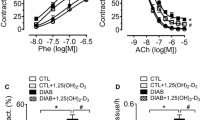
There was no significant difference in aortic vessel contraction to phenylephrine between diabetic and non-diabetic mice (Fig. 1). However, endothelium-dependent relaxation to acetylcholine, the receptor-mediated eNOS agonist, was significantly decreased in diabetic mice compared with in non-diabetic controls (n=7, p<0.05). Endothelium-independent relaxation to sodium nitroprusside, a direct NO donor, was similar in the two groups. These results suggest that impaired vessel relaxation in diabetes is endothelium dependent.
Impaired aortic vessel relaxation in diabetic Balb/c mice. a Contraction of the aortic rings in non-diabetic (open circles) and diabetic (filled circles) mice in response to different concentrations of phenylephrine. b Endothelial-dependent relaxation of the aortic rings in non-diabetic (open circles) and diabetic (filled circles) mice in response to different concentrations of acetylcholine (*p<0.05, n=7). c Endothelial-independent relaxation of the aortic rings in non-diabetic (open circles) and diabetic (filled circles) mice in response to different doses of sodium nitroprusside. Data are means±SEM of seven animals
We next assessed the functional relationships of NO bioavailability and endothelial function in diabetic and non-diabetic mice. EPR quantification of the NO-Fe(DETC)2 adduct revealed a 50% (n=5, p<0.05) decrease in NO production in diabetic mice (Fig. 2) compared with in non-diabetic mice, demonstrating a loss of NO bioavailability in diabetic aorta.
Decreased NO production in diabetic Balb/c mouse aortas. The aortas were stimulated with A23187 (1 μmol/l) for 90 min. eNOS activity was determined by measuring NO–Fe(DETC)2 signal from the extracts. Data are means±SEM of five animals and are normalised to the dry weight of the aorta in milligrams (arbitrary unit/mg) (*p<0.05, n=5)
Superoxide production in the diabetic aorta of the Balb/c mouse
We investigated the effect of diabetes on vascular oxidative stress using two complementary methods. Lucigenin-enhanced chemiluminescence assays showed a doubling of SO production in diabetic animals (n=5, p<0.05) (Fig. 3a). In line with this finding, we observed greatly increased dihydroethidine fluorescence throughout all layers of the vessel wall in diabetic aorta when visualised at low (×10) or high (×60) magnification (Fig. 3b).
Increased SO production in diabetic Balb/c mouse aortas. a The aortas were gassed with 95% oxygen/5% carbon dioxide in warmed Krebs–HEPES buffer for 30 min. SO production was measured using 10 μmol/l lucigenin-enhanced chemiluminescence. Data are means±SEM of five animals and are normalised to the dry weight of the aorta in milligrams (arbitrary unit/mg) (*p<0.05, n=5). RLU, relative light units. b The aortic sections were incubated with dihydroethidine (2.0 μmol/l) for 10 min. Fluorescence intensity (red) in the diabetic mice was stronger than in the non-diabetic mice (×10 magnification). Endothelial cells on the luminal side of the internal elastic lamina with ethidium fluorescence (white arrows) are indicated (×60 magnification), while elastic laminae exhibit green autofluorescence. Typical observations are shown
Biopterin levels in the diabetic aorta of the Balb/c mouse
We assessed the relationship between increased SO production and biopterin levels, in particular BH4 bioavailability. Total biopterin and BH4 levels, as well as the ratio of BH4: total biopterins in diabetic animals, were significantly lower than those in non-diabetic controls (Table 1), suggesting oxidation of BH4 with an increase in BH2 formation.
eNOS expression and dimerisation in the diabetic aortas of Balb/c and C57BL/6 mice
We assessed the effects of diabetes on eNOS protein expression and dimerisation in two strains of mouse, Balb/c and C57BL/6, treated with STZ or STZ vehicle buffer. As expected, total eNOS protein levels increased 1.5-fold (n=6, p<0.05) in diabetic mice relative to controls, as assayed by western blotting in the two mouse strains (Fig. 4a,b). In contrast, the ratio of eNOS dimer:monomer in diabetes was only half that in the controls (n=6, p<0.05), as assayed by low-temperature SDS-PAGE and immunoblotting (Fig. 4a,c).
eNOS protein expression and dimerisation in the diabetic aortas of Balb/c and C57BL/6 mice. a Boiled or non-boiled vessel lysates were fractionated by SDS-PAGE or low-temperature SDS-PAGE, respectively, and immunoblotted with monoclonal antibody to eNOS. b Plot of eNOS bands intensity (*p<0.05, n=6). c Plot of ratio of eNOS bands intensity (dimer: monomer) (*p<0.05, n=6)
eNOS expression and dimerisation in the diabetic aorta of the GCH-Tg mouse
To investigate more directly the role of BH4 in eNOS regulation, we assayed eNOS expression and dimerisation by immunoblotting in the aortas of diabetic GCH-Tg mice. In this mouse strain, sustained levels of BH4 maintain aortic vasorelaxation and eNOS enzymatic activity despite the induction of diabetes by STZ [22]. We observed no difference in eNOS expression between diabetic GCH-Tg mice and their wild-type (C57BL/6) littermates (Fig. 5). However, eNOS dimer was preserved in the diabetic GCH-Tg mouse. Densitometric quantification of multiple blots showed that the ratio of eNOS dimer : monomer in diabetic GCH-Tg mice was 1.8-fold greater than that in diabetic wild-type mice (n=3, p<0.05) (Fig. 5b,c). These results demonstrate that selectively augmenting BH4 levels by targeted overexpression of GTPCH in endothelial cells in vivo preserves eNOS dimerisation.
eNOS protein expression and dimerisation in the diabetic aortas of GCH transgenic mice. a Boiled or non-boiled vessel lysates were fractionated by SDS-PAGE or low-temperature SDS-PAGE, respectively, and immunoblotted with monoclonal antibody to eNOS. b Plot of eNOS bands intensity (NS, n=6). c Plot of ratio of eNOS bands intensity (dimer : monomer) (*p<0.05, n=6)
Discussion
In the present study, we investigated the association between BH4 bioavailability and eNOS function, particularly the protein dimerisation, in STZ-induced diabetic mice. We observed that loss of BH4 in the diabetic aorta was related to impaired vasomotor function, characterised by a decrease in NO production and a concomitant increase in SO production. Furthermore, eNOS expression in the diabetic aorta was up-regulated, but the protein mainly existed in an inactive monomeric form. We also observed that augmenting intracellular BH4 by specific overexpression of GTPCH in the endothelium preserved eNOS dimerisation in the STZ-induced diabetic aorta.
Previous studies have shown that STZ-induced hyperglycaemia impairs aortic endothelium-dependent vessel relaxation [24], accompanied by an increase in SO production [4, 27, 28], and appears to be associated with loss of BH4 in the aorta [22]. Our present study confirmed these findings, showing that hyperglycaemia results in impaired aortic vessel relaxation and decreased NO bioavailability, but with concurrent increased SO production. The increased dihydroethidine fluorescence was present in all layers of the aortic wall, suggesting the presence of other important sources of SO production in diabetes; the importance of the NADPH oxidases in vascular superoxide production in smooth muscle cells and the adventitia is known [4].
BH4 bioavailability is postulated to be limiting in diabetic vascular disease [29]. Loss of BH4 can uncouple eNOS synthesis [17]. It is hypothesised that in this uncoupled state, electron flow from the reductase domain to the oxygenase domain of eNOS is diverted to molecular oxygen rather than l-arginine [6, 30]. This leads to preferential SO production over NO production. Increased SO not only degrades NO, but also forms peroxynitrite [28], a potent oxidant that can rapidly oxidise BH4 to BH3+ and subsequently to BH2 [5, 31]. BH2 may compete with BH4 for eNOS binding, resulting in further impaired eNOS bioactivity [32].
The form of all NOS catalytic activity appears to be homodimer [33–35]. Structural studies suggest a role for BH4 in NOS dimerisation based on purified recombinant proteins in reconstituted cell-free systems [36, 37]. A study based on bovine eNOS expressed in Escherichia coli suggested that BH4 affected eNOS homodimerisation [34]. In another study of recombinant eNOS purified from a baculovirus system, exogenously added BH4 increased eNOS activity and dimerisation [38]. Our observation of increased eNOS protein expression in diabetes is consistent with other reports [4]. Furthermore, with the assay of low-temperature SDS-PAGE, we found eNOS protein in tissue lysates to be mainly present in the inactive monomeric form in the diabetic aorta. Whether these observations reflect unstable eNOS homodimer owing to BH4 deficiency in vivo remains to be investigated. Indeed, techniques to directly study eNOS dimerisation in living cells and tissues are currently lacking. Some recent studies suggest that the neuronal NOS homodimer is more stable than free monomers, as monomers are more readily degraded through the ubiquitin–proteasome pathway [39, 40]. However, the finding that total eNOS protein is increased in diabetes suggests that unstable eNOS homodimerisation, leading to eNOS degradation, is unlikely to be the major mechanism mediating the effects of BH4 on endothelial function in diabetes.
The role of BH4 in the regulation of eNOS dimerisation remains controversial. One structural study based on recombinant eNOS and native eNOS of bovine aortic endothelial cells cultured in high glucose suggested that dissociation of eNOS dimers to monomers was a result of peroxynitrite oxidation on the zinc-thiolate cluster where the BH4 binding site is located [28] and of nitrosylation on the zinc-thiolate cluster at the dimeric interface [41]. After peroxynitrite oxidation of the zinc-thiolate cluster, supplementation with a high dose (100 μmol/l) of BH4 did not prevent dissociation or reassemble the dissociated eNOS dimers [28]. However, findings from another study are not consistent with this observation, showing that dysfunctional eNOS in bovine aortic endothelial cells subjected to peroxynitrite was fully restored by supplementation with BH4 when EPR was used to measure NO production [31]. Indeed, these experiments based on supplementation with high doses of extracellular BH4 may result in some unpredictable effects. This redox-active compound can be pro-oxidant, directly leading to superoxide generation that reduces NO bioactivity [42, 43]; it can also be anti-oxidant by simply removing the free radical species, i.e. peroxynitrite, rather than having any specific effect on NOS activity or regulation [5, 6, 31, 44].
We previously showed that in the GCH-Tg transgenic mouse model, specific human GTPCH overexpression in endothelium by tie-2 promoter control displayed augmenting BH4 levels in vascular tissues [22]. We further demonstrated that STZ-induced diabetic GCH-Tg mice maintained the levels of BH4 for aortic vasorelaxation and eNOS coupling seen in the diabetic wild-type mice. Thus, the diabetic GCH-Tg mouse is an ideal model for investigating the association of BH4 bioavailability with eNOS dimerisation in diabetes.
In the present experiments, we found that GTPCH overexpression in diabetic aortic endothelium resulted in significant preservation of eNOS dimer, which shows that augmenting intracellular BH4 maintains stability of eNOS dimerisation. Our data thus support a view that the principal action of BH4 in diabetic endothelium is to regulate eNOS normal enzymatic activity. This is accompanied by promotion of eNOS homodimerisation. Our findings in diabetic aortic endothelium suggest that under oxidative stress, the integrity of the zinc-thiolate complex of eNOS requires BH4 coordination.
Endothelial dysfunction is an early feature of diabetic vascular disease and BH4 deficiency is postulated to be partially responsible for this. Increasing BH4 synthesis has been shown to restore eNOS function in diabetes [4]. GTPCH overexpression specific to vascular endothelium appears to maintain eNOS coupling as well as vasorelaxation in STZ-induced diabetic mice [22]. We now show that in the diabetic state, expression of eNOS is up-regulated but mainly exists in a monomeric form. Augmented intracellular BH4 by specific GTPCH overexpression can preserve eNOS protein dimerisation, an active protein structure. Thus, increasing endothelial BH4 and/or protecting it from oxidation may be a rational therapeutic strategy to restore eNOS function in diabetes.
Abbreviations
- BH4:
-
tetrahydrobiopterin
- DETC:
-
diethyldithiocarbamate
- eNOS:
-
endothelial nitric oxide synthase
- EPR:
-
electron paramagnetic resonance
- GTPCH:
-
GTP cyclohydrolase
- GCH-Tg:
-
GTPCH transgenic
- NO:
-
nitric oxide
- SO:
-
superoxide
- STZ:
-
streptozotocin
References
Creager MA, Luscher TF, Cosentino F, Beckman JA (2003) Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 108:1527–1532
Harrison DG (1998) Cellular and molecular mechanisms of endothelial dysfunction. J Clin Invest 100:2153–2157
Ishii H, Koya D, King GL (1998) Protein kinase C activation and its role in the development of vascular complications in diabetes mellitus. J Mol Med 76:21–31
Hink U, Li H, Mollnau H et al (2001) Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88:E14–E22
Laursen JB, Somers M, Kurz S et al (2001) Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103:1282–1288
Vasquez-Vivar J, Kalyanaraman B, Martasek P et al (1998) Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A 95:9220–9225
Stuehr D, Pou S, Rosen GM (2001) Oxygen reduction by nitric-oxide synthases. J Biol Chem 276:14533–14536
Stuehr DJ, Griffith OW (1992) Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol 65:287–346
Cosentino F, Luscher TF (1999) Tetrahydrobiopterin and endothelial nitric oxide synthase activity. Cardiovasc Res 43:274–278
Thony B, Auerbach G, Blau N (2000) Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J 347:1–16
Stuehr DJ (1999) Mammalian nitric oxide synthases. Biochim Biophys Acta 1411:217–230
Klatt P, Schmidt K, Lehner D, Glatter O, Bachinger HP, Mayer B (1995) Structural analysis of porcine brain nitric oxide synthase reveals a role for tetrahydrobiopterin and l-arginine in the formation of an SDS- resistant dimer. EMBO J 14:3687–3695
Tzeng E, Billiar TR, Robbins PD, Loftus M, Stuehr DJ (1995) Expression of human inducible nitric oxide synthase in a tetrahydrobiopterin (H4B)-deficient cell line—H4B promotes assembly of enzyme subunits into an active enzyme. Proc Natl Acad Sci U S A 92:11771–11775
Reif A, Frohlich LG, Kotsonis P et al (1999) Tetrahydrobiopterin inhibits monomerization and is consumed during catalysis in neuronal NO synthase. J Biol Chem 274:24921–24929
Cai S, Alp NJ, McDonald D, Canevari L, Heales S, Channon KM (2002) GTP cyclohydrolase I gene transfer augments intracellular tetrahydrobiopterin in human endothelial cells: effects on nitric oxide synthase activity, protein levels and dimerization. Cardiovasc Res 55:838–849
Panda K, Rosenfeld RJ, Ghosh S, Meade AL, Getzoff ED, Stuehr DJ (2002) Distinct dimer interaction and regulation in nitric-oxide synthase types I, II, and III. J Biol Chem 277:31020–31030
Katusic ZS (2001) Vascular endothelial dysfunction: does tetrahydrobiopterin play a role? Am J Physiol Heart Circ Physiol 281:H981–H986
Pieper GM (1997) Acute amelioration of diabetic endothelial dysfunction with a derivative of the nitric oxide synthase cofactor, tetrahydrobiopterin. J Cardiovasc Pharmacol 29:8–15
Meininger CJ, Marinos RS, Hatakeyama K et al (2000) Impaired nitric oxide production in coronary endothelial cells of the spontaneously diabetic BB rat is due to tetrahydrobiopterin deficiency. Biochem J 349:353–356
Shinozaki K, Nishio Y, Okamura T et al (2000) Oral administration of tetrahydrobiopterin prevents endothelial dysfunction and vascular oxidative stress in the aortas of insulin-resistant rats. Circ Res 87:566–573
Heitzer T, Krohn K, Albers S, Meinertz T (2000) Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with type II diabetes mellitus. Diabetologia 43:1435–1438
Alp NJ, Mussa S, Khoo J et al (2003) Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest 112:725–735
Soriano FG, Pacher P, Mabley J, Liaudet L, Szabo C (2001) Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-ribose) polymerase. Circ Res 89:684–691
Soriano FG, Virag L, Szabo C (2001) Diabetic endothelial dysfunction: role of reactive oxygen and nitrogen species production and poly(ADP-ribose) polymerase activation. J Mol Med 79:437–448
Khoo JP, Alp NJ, Bendall JK et al (2004) EPR quantification of vascular nitric oxide production in genetically modified mouse models. Nitric Oxide 10:156–161
Subbanna I, de Baere T, Therasse E, Prade M, Eisele G, Roche A (1993) Experimental study of balloon-expandable metallic vena caval stents in rabbits. J Vasc Interv Radiol 4:753–758
Cosentino F, Hishikawa K, Katusic ZS, Luscher TF (1997) High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 96:25–28
Zou MH, Shi C, Cohen RA (2002) Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest 109:817–826
Landmesser U, Dikalov S, Price SR et al (2003) Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111:1201–1209
Xia Y, Tsai AL, Berka V, Zweier JL (1998) Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem 273:25804–25808
Kuzkaya N, Weissmann N, Harrison DG, Dikalov S (2003) Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278:22546–22554
Vasquez-Vivar J, Duquaine D, Whitsett J, Kalyanaraman B, Rajagopalan S (2002) Altered tetrahydrobiopterin metabolism in atherosclerosis: implications for use of oxidized tetrahydrobiopterin analogues and thiol antioxidants. Arterioscler Thromb Vasc Biol 22:1655–1661
Ghosh DK, Abusoud HM, Stuehr DJ (1996) Domains of macrophage N(O) synthase have divergent roles in forming and stabilizing the active dimeric enzyme. Biochemistry 35:1444–1449
Rodriguez-Crespo I, Gerber NC, Ortiz de Montellano PR (1996) Endothelial nitric-oxide synthase. Expression in Escherichia coli, spectroscopic characterization, and role of tetrahydrobiopterin in dimer formation. J Biol Chem 271:11462–11467
Venema RC, Ju H, Zou R, Ryan JW, Venema VJ (1997) Subunit interactions of endothelial nitric-oxide synthase. Comparisons to the neuronal and inducible nitric-oxide synthase isoforms. J Biol Chem 272:1276–1282
Raman CS, Li H, Martasek P, Kral V, Masters BS, Poulos TL (1998) Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell 95:939–950
Fischmann TO, Hruza A, Niu XD et al (1999) Structural characterization of nitric oxide synthase isoforms reveals striking active-site conservation. Nat Struct Biol 6:233–242
Wever RMF, van Dam T, van Rijn HJ, de Groot F, Rabelink TJ (1997) Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem Biophys Res Commun 237:340–344
Bender AT, Demady DR, Osawa Y (2000) Ubiquitination of neuronal nitric-oxide synthase in vitro and in vivo. J Biol Chem 275:17407–17411
Noguchi S, Jianmongkol S, Bender AT, Kamada Y, Demady DR, Osawa Y (2000) Guanabenz-mediated inactivation and enhanced proteolytic degradation of neuronal nitric-oxide synthase. J Biol Chem 275:2376–2380
Ravi K, Brennan LA, Levic S, Ross PA, Black SM (2004) S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci U S A 101:2619–2624
Tsutsui M, Milstien S, Katusic ZS (1996) Effect of tetrahydrobiopterin on endothelial function in canine middle cerebral arteries. Circ Res 79:336–342
Kinoshita H, Katusic ZS (1996) Exogenous tetrahydrobiopterin causes endothelium-dependent contractions in isolated canine basilar artery. Am J Physiol 271:H738–H743
Milstien S, Katusic Z (1999) Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun 263:681–684
Acknowledgements
This work was supported by grants from the British Heart Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, S., Khoo, J., Mussa, S. et al. Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia 48, 1933–1940 (2005). https://doi.org/10.1007/s00125-005-1857-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-1857-5