Abstract
Aims/hypothesis
The incretin hormone glucagon-like peptide 1 (GLP-1) has antihyperglycaemic effects, but its therapeutic usefulness is limited by its metabolic instability. Dipeptidyl peptidase IV (DPP-IV) is established as the primary inactivating enzyme, but the roles of other enzymes remain unclear.
Methods
The effect of candoxatril, a selective inhibitor of neutral endopeptidase (NEP) 24.11, on GLP-1 pharmacokinetics/pharmacodynamics with or without DPP-IV inhibition was examined in anaesthetised pigs.
Results
During GLP-1 infusion, candoxatril doubled C-terminal immunoreactivity, improving the pharmacokinetics (t ½ 2.3±0.1 to 8.8±1.2 min; metabolic clearance rate [MCR] 20.4±3.4 to 4.8±0.4 ml·kg−1· min−1; p<0.01), but had no effect upon intact GLP-1 (t ½ 1.4±0.1 to 1.6±0.1 min; MCR 47.9±8.0 to 38.8±5.0 ml·kg−1·min−1). Insulin responses to intravenous glucose were unaffected by candoxatril, but glucose tolerance was improved (ΔAUCmin 27–87 118±5 to 74±14 min·mmol·l−1; glucose elimination rate [k] 6.6±0.5 to 8.6±0.5%; p<0.05). When candoxatril was co-administered with valine pyrrolidide (a DPP-IV inhibitor), changes in C-terminal GLP-1 pharmacokinetics mirrored those seen when candoxatril alone was administered (t ½ 2.7±0.3 and 7.7±0.8 min; MCR 17.3±2.6 and 6.5±0.8 ml·kg−1·min−1 for valine pyrrolidide without and with candoxatril, respectively). However, intact GLP-1 pharmacokinetics were improved (t ½ 2.8±0.3 and 7.5±0.6 min; MCR 18.3±0.6 and 9.4±0.9 ml·kg−1·min−1; p<0.02), potentiating the antihyperglycaemic/insulinotropic effects of GLP-1 (glucose ΔAUCmin 27– 87 103±8 to 62±14 min·mmol·l−1; k 6.8±0.4 to 11.4±1.4%; insulin ΔAUCmin 27–87 3,680±738 to 7,201±1,183 min·pmol·l−1; p<0.05).
Conclusions/interpretation
This study confirms a role for NEP-24.11 in GLP-1 metabolism in vivo, suggesting that up to 50% of GLP-1 entering the circulation may be degraded by NEP-24.11. Furthermore, combined inhibition of DPP-IV and NEP-24.11 is superior to DPP-IV inhibition alone in preserving intact GLP-1, which raises the possibility that the combination has therapeutic potential.
Similar content being viewed by others
Introduction
Glucagon-like peptide 1 (GLP-1) has a spectrum of effects, which makes it an attractive target in the search for new therapies for type 2 diabetes [1]. However, a major factor limiting clinical application is its metabolic instability, as it is rapidly degraded and inactivated in vivo [2–4]. Strategies that take advantage of the beneficial effects of GLP-1 include the development of degradation-resistant analogues and selective enzyme inhibitors to enhance the effects of the endogenously released peptide [3, 5], and recent research has focused on GLP-1 degradation in vivo in an attempt to identify the enzymes with physiological relevance.
Structure–activity studies have shown that residues in the C-terminal region of the peptide are important for receptor binding, while the N-terminal dipeptide is crucial for receptor activation [6]. Consequently, most studies investigating GLP-1 metabolism have addressed the mechanisms behind the N-terminal truncation, as this seems to be the primary inactivating step. In vitro studies have revealed that GLP-1 is a substrate for dipeptidyl peptidase IV (DPP-IV) [7], while subsequent studies in vivo suggest that DPP-IV is likely to play a role in regulating its metabolic stability [2–4]. This was confirmed by the finding that N-terminal cleavage of exogenous and endogenous GLP-1 is practically eliminated by DPP-IV inhibition in vivo [8–10] and that endogenous GLP-1 stability is improved in mice lacking DPP-IV catalytic activity [11]. Several studies have shown that DPP-IV inhibitors improve glucose tolerance in rodents [8, 12–14] and patients with type 2 diabetes [15, 16]; these inhibitors are now in the late stages of clinical development as antihyperglycaemic agents.
Fewer studies have examined the involvement of other enzymes in GLP-1 degradation in vivo. Neutral endopeptidase 24.11 (NEP-24.11, also known as neprilysin; see Turner et al. [17] for review) is a widespread membrane-bound zinc metallopeptidase with a broad substrate specificity. It is found in high concentrations in the kidney, where it may be involved in the renal clearance of peptide hormones. NEP can degrade members of the glucagon/secretin/glucose-dependent insulinotropic polypeptide (GIP) family of peptides, including GLP-1, in vitro [18, 19], but the significance of this observation in terms of GLP-1 degradation in vivo is unknown.
This study investigated the physiological relevance of NEP-24.11 in GLP-1 metabolism, using candoxatril (a selective NEP-24.11 inhibitor [20, 21]) and a previous protocol that revealed the physiological role of DPP-IV in GLP-1 and GIP metabolism [9, 22] and of NEP-24.11 in glucagon degradation [23]. The influence of candoxatril on the metabolic stability and antihyperglycaemic/insulinotropic effects of GLP-1 was examined in anaesthetised pigs. In addition, these parameters were examined with DPP-IV inhibition alone and with DPP-IV inhibition together with candoxatril, to see whether inhibition of NEP-24.11 leads to further improvement beyond that achieved by DPP-IV inhibition alone. Findings from part of this study (preliminary data; effects of combined DPP-IV/NEP inhibition) have previously been reported in brief [24].
Materials and methods
Animals and surgical procedures
Animal studies were in accordance with international guidelines (National Institutes of Health publication no. 85-23, revised 1985, and Danish legislation governing animal experimentation, 1987) and were carried out after permission had been granted by the Animal Experiments Inspectorate, Ministry of Justice, Denmark.
Non-fasted Danish LYY strain pigs (30–35 kg) were used for study. After premedication with midazolum (0.5 mg/kg, Dormicum; Roche, Basel, Switzerland) and ketamine (10 mg/kg, Ketaminol; Veterinaria, Zurich, Switzerland), animals were anaesthetised with i.v. α-chloralose (66 mg/kg; Merck, Darmstadt, Germany) and ventilated with intermittent positive pressure using N2O/O2. Catheters were placed in the right carotid artery for sampling of arterial blood, in the left ear vein for peptide infusion and in the right ear vein for valine pyrrolidide (a selective DPP-IV inhibitor [25]) and/or candoxatril administration. After surgical preparation, animals were heparinised and left undisturbed for 30 min. Anaesthesia was maintained with additional chloralose as necessary.
The experimental protocol is outlined in Fig. 1. In six animals, two basal arterial blood samples (3 ml) were collected, after which 20 ml of vehicle (0.9% NaCl), was given as a bolus over 2 min via an ear vein. After 10 min, the first of two identical infusions of GLP-1 (7-36)amide (Bachem, Bubendorf, Switzerland; dissolved in 0.9% NaCl containing 1% human serum albumin; Calbiochem; VWR International, Albertslund, Denmark) was started at a rate of 0.75 pmol·kg−1·min−1 for 47 min using a syringe pump, commencing at time 0. An intravenous glucose infusion (0.2 g/kg; 50% solution) was administered from the 28th to the 37th minute. Arterial blood samples were taken throughout the GLP-1 infusion period and for 60 min after it had ended (Fig. 1). Then, candoxatril (5 mg/kg, dissolved in 0.9% NaCl), a selective NEP-24.11 inhibitor [20, 21] (kindly provided by Dr Jane Lundbeck, Novo Nordisk, Måløv, Denmark) was given as a bolus i.v. injection via the ear vein catheter over 2 min. After 30 min, the second GLP-1 infusion was started. The protocol was repeated for blood sampling and glucose infusion. The blood volume taken over the entire procedure was approximately 5% of the total blood volume, and this fluid loss was replaced by flushing the catheters with 5 ml 0.9% NaCl after each sample was taken. This protocol has previously been shown to have no effect on blood pressure or heart rate [26] or on the metabolic clearance of a second compared with a first infusion of GLP-1 [9] or the related peptide GIP [22], both given without enzyme inhibition.
Schematic outline of the experimental protocol. Blood samples (3 ml) were taken from the carotid artery at the points indicated by the inverted triangles. The vertical arrows show when vehicle (0.9% NaCl) or valine pyrrolidide (val-pyd; 300 μmol/kg) and candoxatril (Candox; 5 mg/kg) were administered. The periods of GLP-1 infusion (0.75 pmol·kg−1·min−1) and intravenous glucose administration (G; 0.2 g/kg) are represented by the two lower horizontal boxes, while the two upper horizontal boxes indicate the time periods during which the AUCs for GLP-1, glucose and insulin were calculated
In separate experiments (n=7), the effect of combined DPP-IV/NEP-24.11 inhibition was examined. The protocol above was repeated, except that vehicle was replaced by valine pyrrolidide (300 μmol/kg, a dose previously shown to inhibit plasma DPP-IV activity by >95% for at least 150 min [9, 22]; kindly provided by Dr L Christensen, Novo Nordisk) prior to the first GLP-1 infusion. Animals received candoxatril prior to the second GLP-1 infusion, as in the previous experiment.
Because of the long survival time of both inhibitors, these experiments could not be carried out as conventional cross-over studies. Therefore, to ensure that any additional effect of candoxatril was not simply a result of the inhibitor combination always being given prior to the second GLP-1 infusion, a control experiment (n=4) was performed. The protocol above was repeated, with animals receiving valine pyrrolidide prior to the first GLP-1 infusion and vehicle (0.9% NaCl) instead of candoxatril prior to the second GLP-1 infusion.
Blood glucose was measured immediately (One Touch II; Lifescan, Lyngby, Denmark). Blood samples were collected into chilled tubes containing EDTA (7.4 mmol/l final concentration) and valine pyrrolidide (0.01 mmol/l final concentration) for hormonal analysis, and into heparinised tubes for measurement of NEP-24.11 enzyme activity. Samples were kept on ice until centrifugation at 4°C. Plasma was separated and stored at −20°C until analysis.
Hormonal assays
Plasma samples were assayed for GLP-1 using antiserum 89390 [27], which has an absolute requirement for the intact amidated C terminus of the molecule and cross-reacts less than 0.01% with C-terminally truncated fragments or glycine-extended GLP-1 [GLP-1 (7-37)] and 83% with GLP-1 (9-36)amide. Plasma was extracted with 70% ethanol (v/v, final concentration) before assay, giving a recovery of added GLP-1 of 75% [28]. Intact GLP-1 concentrations were measured in unextracted plasma by two-site (sandwich) ELISA using a catcher monoclonal antibody (GLP1F5) specific for the C terminus and a detecting monoclonal antibody (Mab26.1) specific for the intact N terminus [29]. This assay shows less than 0.1% cross-reactivity with N-terminally extended or truncated forms of GLP-1 and less than 0.1% cross-reactivity with forms truncated by more than three residues from the C terminus. Insulin immunoreactivity was measured in unextracted plasma using antiserum 2004 [28], and glucagon immunoreactivity was measured after ethanol extraction using the C-terminally directed antiserum 4305, which measures glucagon of pancreatic origin [28].
Measurement of NEP-24.11 activity
NEP-24.11 activity was assessed using modified assay conditions from a previously published method [30], as described in previous work [23]. Briefly, plasma was diluted nine-fold in 0.9% NaCl, and 50 μl was added to a quartz cuvette (1-cm path) with 20 μl aminopeptidase M containing 4 μg enzyme protein (0.096 U; Sigma). The reaction was initiated by adding 280 μl substrate (0.262 μmol in 0.05 mol/l Tris buffer, pH 7.5; Bachem). Samples were incubated at 25°C and the absorbance (405 nm) was read using a spectrophotometer after 30 min.
Calculations and statistical analysis
During GLP-1 infusion, a plateau in arterial GLP-1 levels was achieved after 20 min. The plateau concentration was therefore defined as the mean of the last four measurements during the GLP-1 infusion. The plasma t ½ was calculated by loge-linear regression analysis of peptide concentrations following termination of the infusion (after subtraction of endogenous arterial GLP-1 concentrations). The metabolic clearance rate (MCR) was calculated using the following formula:
where IR indicates immunoreactivity. The area under the GLP-1 curves was calculated using the trapezoidal method. For glucose and insulin, incremental areas (ΔAUC) were calculated from the start of i.v. glucose until 40 min after the end of the GLP-1 infusion (i.e. min 27–87), after subtraction of the concentration in the 25-min sample taken before glucose administration. Fractional clearances (k) for glucose were calculated immediately following the glucose infusion until the end of the GLP-1 infusion (i.e. min 37–47) using the formula k=0.693/t ½.
Data are expressed as means±SEM and were analysed using GraphPAD InStat software, version 3.05 for Windows 95 (San Diego, CA, USA) and Statistica software (StatSoft, Tulsa, OK, USA). The t ½, the AUCs and the MCR were analysed using ANOVA (where appropriate) and two-tailed t-tests for paired and non-paired data. A p value of less than 0.05 was considered significant.
Results
NEP-24.11 activity
Plasma samples collected before and after in vivo candoxatril administration were tested for NEP-24.11 activity, with results expressed as a percentage of the NEP-24.11 activity (absorbance) in the basal (before candoxatril administration in vivo) samples. Plasma samples collected 30 min after in vivo candoxatril administration (immediately before the start of the second GLP-1 infusion) contained enough inhibitor to halt NEP-24.11 activity in vitro by 74.4±1.8%. The percentage gradually decreased to 60.2±2.4% by the end of the peptide infusion, reaching 46.1±3.7% in samples collected at the end of the experiment. A similar percentage of inhibition was obtained when candoxatril was administered after valine pyrrolidide.
Effects of candoxatril alone
The effects of candoxatril alone were examined in six animals. When GLP-1 alone was infused, the overall AUC during and after the infusion (AUCmin 0–107) for C-terminal immunoreactivity was 2,634±745 min·pmol·l−1. This was increased by candoxatril (to 7,011±1,697 min·pmol·l−1, p=0.0109; Fig. 2). Accordingly, the metabolic stability measured by C-terminal assay was greatly improved (t ½ from 2.3±0.1 to 8.8±1.2 min, p<0.0006; MCR from 20.4±3.4 to 4.8±0.4 ml·kg−1·min−1, p=0.0071). In contrast, candoxatril had no effect on any parameter measured by ELISA for intact GLP-1 (AUCmin 0–107 from 2,170±159 to 2,181±277 min·pmol·l−1; t ½ from 1.4±0.1 to 1.6±0.1 min; MCR from 47.9±8.0 to 38.8±5.0 ml·kg−1·min−1; all p>0.2).
Plasma GLP-1 immunoreactivity in the carotid artery, measured by C-terminal RIA (black squares) and two-site ELISA for intact GLP-1 (black triangles). Animals received 47-min infusions of GLP-1 (0.75 pmol·kg−1·min−1), given alone and with NEP-24.11 inhibition. Candoxatril (Candox; 5 mg/kg) was given 60 min after the end of the first GLP-1 infusion, and after a further 30 min the second GLP-1 infusion was initiated. Data are means±SEM; n=6. Horizontal arrows indicate infusion periods
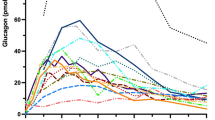
Previous studies [9] have revealed no differences in blood glucose or insulin responses to a first vs a second GLP-1/glucose infusion. In the present study, the presence of candoxatril during the second GLP-1 infusion reduced the glucose excursion compared with during the infusion of GLP-1 alone (Fig. 3a; ΔAUCmin 27–87 118±5 to 74±14 min·mmol·l−1, p=0.0474), and the rate of glucose elimination increased (k 6.6±0.5 to 8.6±0.5%/min, GLP-1 alone vs GLP-1+candoxatril; p=0.0478). Insulin concentrations increased following glucose administration but were unaffected by candoxatril (Fig. 3b; ΔAUCmin 27–87 3,499±514 vs 3,364±695 min·pmol·l−1, p=0.822). Glucagon concentrations increased after candoxatril administration and remained elevated (Fig. 3c).
Blood glucose (a), plasma insulin (b) and plasma glucagon (c) concentrations measured before, during and after 47-min infusions of GLP-1 (0.75 pmol·kg−1·min−1), given alone (min 0–107) and with NEP-24.11 inhibition (min 107–244). Candoxatril (Candox; 5 mg/kg) was given 60 min after the end of the first GLP-1 infusion, and after a further 30 min the second GLP-1 infusion was initiated. Intravenous glucose (0.2 g/kg) was administered from the 28th to the 37th minute of each GLP-1 infusion. Data are means±SEM; n=6. Horizontal arrows indicate infusion periods
Comparison of DPP-IV inhibition alone and combined DPP-IV/NEP-24.11 inhibition
A study of valine pyrrolidide administration alone and combined with candoxatril was carried out in seven animals, but owing to some sample loss there was only sufficient plasma to measure GLP-1 concentrations with both assays in four animals. During the first infusion with DPP-IV inhibition alone, N-terminal GLP-1 degradation was prevented and the pharmacokinetic parameters measured by both assays were the same (AUCmin 0–107 3,656±692 vs 3,387±679 min·pmol·l−1; t ½ 2.7±0.3 vs 2.8±0.3 min; MCR, 17.3±2.6 vs 18.3±0.6 ml·kg−1·min−1; C-terminal assay vs ELISA, all p>0.2). Co-administration of candoxatril increased GLP-1 concentrations measured by both assays (p<0.03 for each assay, relative to valine pyrrolidide alone), and although there was a tendency for levels measured by C-terminal assay to exceed those measured by ELISA, this was not significant (AUCmin 0–107 6,293±614 vs 4,724±645 min·pmol·l−1, C-terminal vs ELISA, p=0.180). Accordingly, the t ½ was prolonged by the inhibitor combination (C-terminal assay to 7.7±0.8 min, p=0.003; ELISA to 7.5±0.6 min, p=0.001) and the MCR was reduced (C-terminal assay to 6.5±0.8 ml·kg−1·min−1, p=0.023; ELISA to 9.4±0.9 ml·kg−1·min−1, p=0.013). Overall, plasma profiles resembled the C-terminal plasma GLP-1 levels shown in Fig. 2.
Data for GLP-1 pharmacodynamics are presented for all seven animals. The improvement in the metabolic stability of GLP-1 was associated with improved antihyperglycaemic and insulinotropic effects. Thus, compared with the first GLP-1 infusion with valine pyrrolidide alone, k glucose was increased by co-administration of candoxatril (from 6.8±0.4 to 11.4±1.4 %/min; p=0.012), resulting in a smaller glucose excursion following intravenous glucose (Fig. 4a; ΔAUCmin 27–87 103±8 vs 62±14 min·mmol·l−1, p=0.0404).
Blood glucose (a), plasma insulin (b) and plasma glucagon (c) concentrations measured before, during and after 47-min infusions of GLP-1 (0.75 pmol·kg−1·min−1), given with DPP-IV inhibition alone (min 0–107) and together with NEP-24.11 inhibition (min 107–244). The first GLP-1 infusion was started 20 min after administration of valine pyrrolidide (Val-pyd; 300 μmol/kg). Candoxatril (Candox; 5 mg/kg) was given 60 min after the end of the first GLP-1 infusion, and after a further 30 min the second GLP-1 infusion was initiated. Intravenous glucose (0.2 g/kg) was administered from the 28th to the 37th minute of each GLP-1 infusion. Data are means±SEM; n=7. Horizontal arrows indicate infusion periods
Insulin concentrations in six of the seven animals were increased by adding candoxatril, but in one animal, they were slightly lower. Accordingly, when all seven animals were included (Fig. 4b), although the mean AUC was increased by candoxatril (ΔAUCmin 27–87 3,974±689 vs 6,542±1,197 min·pmol·l−1), this failed to reach significance (p=0.130, paired t-test). However, when the one non-responder was excluded, the increase in insulin secretion became significant (ΔAUCmin 27–87 3,680±738 vs 7,201±1,183 min·pmol·l−1, p=0.044).
With valine pyrrolidide alone, plasma glucagon concentrations were modestly suppressed by infusion of GLP-1, even before the glucose infusion, and they decreased further once glucose was given, before returning to basal levels (Fig. 4c). Once candoxatril was administered, glucagon concentrations increased more than two-fold in the period before the glucose challenge. After the glucose load, the glucagonostatic effect of GLP-1 was potentiated, but on cessation of the GLP-1 infusion, glucagon levels were restored.
Comparison of two successive GLP-1 infusions during DPP-IV inhibition
As described above, valine pyrrolidide prevents N-terminal GLP-1 degradation, giving similar pharmacokinetic profiles irrespective of which GLP-1 assay is used. However, to verify that changes in pharmacodynamics during the second GLP-1 infusion with both inhibitors were a result of elevated GLP-1 levels rather than of the inhibitor combination only being given with the second GLP-1/glucose infusions, a control experiment was performed. Animals received two successive GLP-1/glucose infusions during DPP-IV inhibition alone, revealing identical GLP-1 pharmacokinetics (AUCmin 0–107 2,613±639 vs 2,798±337 min·pmol·l−1, p=0.8335, 1st vs 2nd infusion; t ½ 2.6±0.3 vs 2.4±0.2 min, p=0.078; MCR 24.5±4.8 vs 24.1±6.2 ml·kg−1·min−1, p=0.9660). Furthermore, the pharmocodynamic responses to the two infusions were similar, with no differences in the profiles of glucose (Fig. 5a; k 7.7±0.3 vs 7.9±0.3 %/min; ΔAUCmin 27–87 81±3 vs 81±17 min·mmol·l−1; both p>0.3), insulin (ΔAUCmin 27–87 2,613±639 vs 2,798±337 min·pmol·l−1, p=0.8335; Fig. 5b) or glucagon (data not shown).
Blood glucose (a) and plasma insulin (b) concentrations in control experiments. Animals received two successive 47-min infusions of GLP-1 (0.75 pmol·kg−1·min−1), given with DPP-IV inhibition. The first GLP-1 infusion was started 20 min after, and the second infusion 157 min after, administration of valine pyrrolidide (300 μmol/kg). Intravenous glucose (0.2 g/kg) was administered from the 28th to the 37th minute of each GLP-1 infusion. Data are means±SEM; n=4. Horizontal arrows indicate infusion periods
Discussion
This study investigated the role of the enzyme NEP-24.11 in GLP-1 metabolism in vivo using the selective inhibitor candoxatril. GLP-1 degradation is reduced by candoxatril, thereby confirming a physiological role for NEP-24.11 in its metabolism. However, the possibility that this may be affected by the anaesthesia cannot be ruled out.
Candoxatril is the pro-drug of the active compound candoxatrilat and is a potent competitive and selective inhibitor of NEP-24.11; it was developed as a diuretic for clinical use [31–33]. Candoxatril does not affect the activity of carboxypeptidase A, leucine aminopeptidase (aminopeptidase M) or angiotensin-converting enzyme, which belong to the same zinc metalloprotease superfamily as NEP-24.11 [20], or that of the more closely NEP-related enzyme, endothelin-converting enzyme-1 [31]. Following oral administration, candoxatril is rapidly absorbed and converted to the active drug by plasma esterases [21, 34]. The effective dose in pigs is unknown, but the chosen dose successfully inhibited glucagon degradation in another study using a similar protocol [23]. In the present study, plasma samples contained enough active inhibitor to inhibit NEP-24.11 activity in the in vitro assay by 50 to 75% (depending on the time elapsing after in vivo candoxatril administration). However, this underestimates the true in vivo inhibition, because the plasma (and hence the inhibitor) was diluted 63-fold in the activity assay, meaning that inhibitor concentrations in vitro were much lower than the original concentrations in vivo, and because the assay requires the addition of a substrate, which competes with the inhibitor for binding to the enzyme.
In vitro studies indicated that NEP-24.11 can cleave GLP-1, and six potential cleavage sites in the central and C-terminal regions were identified. Hydrolysis occurred particularly readily between Glu27-Phe28 and Trp31-Leu32, and other sites included Asp15-Val16, Ser18-Tyr19, Tyr19-Leu20 and Phe28-Ile29 [19]. Cleavage at these sites would generate fragments that are not recognised by either of the GLP-1 assays used in the present study. During infusion of GLP-1 in the presence of candoxatril, C-terminal immunoreactive GLP-1 concentrations doubled and the plasma half-life increased three-fold, suggesting that NEP-24.11 is responsible for the degradation of half of the total amount of GLP-1 in the plasma. It has previously been suggested that while N-terminally directed assays should be used for measuring biologically active, intact GLP-1 concentrations, C-terminally directed assays (detecting both intact GLP-1 and the N-terminally truncated metabolite arising from DPP-IV-mediated degradation) are more suitable for the measurement of total GLP-1 secretion [35]. In light of the findings of the present study, it could now be speculated that current measurements of endogenous GLP-1 secretion, measured by C-terminally directed assays, underestimate the true secretion by a factor of two. With candoxatril alone, cleavage by NEP-24.11 is prevented but DPP-IV is still active, leading to an accumulation of the N-terminally truncated metabolite GLP-1 (9-36)amide. Indeed, levels of intact GLP-1 were not significantly affected by candoxatril alone. However, when the two inhibitors were co-administered, valine pyrrolidide completely prevented N-terminal GLP-1 degradation, and the metabolic stability of intact GLP-1 was improved, resulting in a six-fold increase in its plasma half-life.
The relatively slow clearance of candoxatril (1.9 ml·kg−1·min−1 in humans, 15 ml·kg−1·min−1 in rats [21]) precluded a cross-over experimental design in which the order of inhibitor administration was alternated, raising the possibility that any changes in glucose or insulin concentrations after candoxatril were a result of factors other than NEP inhibition. In previous studies using similar protocols, GLP-1 pharmacokinetics/pharmacodynamics in the absence of any enzyme inhibition were similar during two successive GLP-1 infusions [9]. In the present study, fluid loss was replaced, heart rate and blood pressure remained constant throughout the experiment, and the effects of the two GLP-1 infusions in the presence of valine pyrrolidide alone were not significantly different. This suggests that the pharmacodynamic changes seen during the second GLP-1 infusions after candoxatril administration were a result of the effects of candoxatril.
The presence of candoxatril during the GLP-1 infusion increased the elimination rate of glucose by 30%, resulting in a corresponding reduction in the glucose excursion (AUC) compared with during infusion of GLP-1 alone. This cannot be attributed to GLP-1 itself, because levels of biologically active GLP-1 were unaffected, indicating that other factors must be involved. In this context, it is worth noting that, despite lower glycaemia, insulin levels were maintained during the second GLP-1 infusion with candoxatril. This suggests that candoxatril could have increased levels of another insulin secretagogue; otherwise, a lower glucose-induced insulin secretion would be expected. Additionally, a direct effect of candoxatril on insulin itself may also have contributed to the reduced glucose excursion. In early studies involving the purification of NEP-24.11, degradation of labelled insulin β-chain was used to monitor the enzyme activity [36], suggesting that NEP-24.11 can cleave insulin. However, it is unknown whether the intact insulin molecule is also a substrate. Moreover, it has also been reported that thiorphan (a less selective NEP-24.11 inhibitor) potentiates the hypoglycaemic effects of insulin in rats in vivo, indicating that NEP-24.11 could be involved in insulin catabolism [37]. It is, therefore, possible that candoxatril reduced insulin degradation but that this escaped detection with the insulin assay used. Further studies using an insulin assay with a different specificity or using HPLC should be able to resolve this question.
As mentioned above, candoxatril may have affected the degradation of other endogenous NEP substrates, which in turn influenced glucose homeostasis. GIP is a substrate for NEP in vitro, albeit a poor one, with a rate of hydrolysis that is two orders of magnitude slower than that of GLP- 1 [19]. Nevertheless, the possibility cannot be excluded that candoxatril reduced endogenous GIP degradation in vivo, which contributed to the antihyperglycaemic effect, particularly when DPP-IV was simultaneously inhibited. There is also evidence that bradykinin is degraded by NEP [38], and several studies have shown that selective NEP or dual NEP/ACE (vasopeptidase) inhibitors lead to improved insulin sensitivity in obese, insulin-resistant rats [39–42] via a mechanism involving increased activation of the kinin–nitrous oxide pathway [39, 42]. This improvement in whole-body insulin-mediated glucose disposal, effected by NEP inhibition, seemed to be a result of improvements in insulin sensitivity rather than secondary to changes in cardiovascular or renal function, because heart rate, blood pressure, femoral blood flow and GFR were unaltered [40, 43].
Therefore, given that active GLP-1 levels were unaltered by candoxatril alone in the present study, GLP-1-independent mechanisms must have been responsible for the improved glucose tolerance seen after NEP inhibition alone. However, in the presence of both inhibitors, the situation is different. Intact GLP-1 levels, which are already significantly increased by DPP-IV inhibition as reported previously [9], increase by a further 40% owing to the additional effect of the C-terminal stabilisation brought about by candoxatril, and this is enough to increase insulin levels beyond those achieved by DPP-IV inhibition alone. It is, therefore, reasonable to speculate that in the presence of both inhibitors, the effect of increased active GLP-1 levels together with the GLP-1-independent mechanisms discussed above result in the near doubling of the glucose elimination rate and explain why the glucose excursion is almost halved relative to DPP-IV inhibition alone.
Endogenous glucagon levels (measured by C-terminal assay) increased after candoxatril administration, which is consistent with the results of our recent study showing that NEP-24.11 is involved in glucagon degradation in vivo [23]. However, N-terminal glucagon immunoreactivity is unaffected by candoxatril [23], and since an intact N-terminus is required for the biological activity of glucagon [44], it was concluded that NEP-24.11 in itself does not regulate this activity [23]. Therefore, the changes in endogenous glucagon immunoreactivity seen in the present study following candoxatril administration are likely to reflect the accumulation of N-terminally truncated (inactive) glucagon fragments, which are unlikely to have influenced glucose homeostasis.
DPP-IV cleavage is regarded as being responsible for the initial inactivation of GLP-1, but this study has demonstrated that NEP-24.11 also plays a role in the degradation of the intact peptide and the truncated metabolite. DPP-IV inhibition protects intact GLP-1 from N-terminal truncation, leading to improved insulinotropic and antihyperglycaemic activity, and this is further enhanced by concomitant NEP-24.11 inhibition. DPP-IV inhibitors have reached late-phase clinical trials and have shown promising effects in patients with relatively mild type 2 diabetes (HbA1c of ~7.4%) [15, 16]. Their mechanism of action is believed to include preservation of endogenous intact GLP-1 [45], raising the question of whether this will be enough to confer sufficient glucose-lowering efficacy, especially as monotherapy in patients at more advanced stages of the disease, since there will be a limit to the level of increase in plasma levels of the active hormone (dependent on the maximal secretory capacity of the L cell). In diabetic patients, intact GLP-1 accounts for 30 to 50% of endogenous C-terminal GLP-1 immunoreactivity [46], and clinical studies with DPP-IV inhibition have reported increases in intact GLP-1 of approximately two-fold [15]. However, as the present study shows, up to half of the GLP-1 entering the circulation may be degraded by NEP-24.11, suggesting that combining NEP inhibition with DPP-IV inhibition may be able to further increase active GLP-1 levels, thereby potentiating the effects of DPP-IV inhibitor monotherapy. Further studies are warranted to examine the effect of NEP-24.11 inhibition on endogenous GLP-1 levels, particularly in light of reports suggesting that increased intact GLP-1 may, via a feedback mechanism, inhibit its own release [35]. Such studies may also shed further light on the complex physiological control mechanisms involved in regulating active GLP-1 levels, and may help elucidate the endocrine, paracrine and/or neuromodulatory role of this important peptide hormone.
Abbreviations
- DPP-IV:
-
dipeptidyl peptidase IV
- GIP:
-
glucose-dependent insulinotropic polypeptide
- GLP-1:
-
glucagon-like peptide 1
- MCR:
-
metabolic clearance rate
- NEP:
-
neutral endopeptidase
References
Holst JJ (2002) Therapy of type 2 diabetes mellitus based on the actions of glucagon-like peptide-1. Diabetes Metab Res Rev 18:430–441
Deacon CF, Johnsen AH, Holst JJ (1995) Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide which is a major endogenous metabolite in vivo. J Clin Endocrinol Metab 80:952–957
Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ (1995) Both subcutaneously and intravenously administered glucagon-like peptide-1 are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 44:1126–1131
Kieffer TJ, McIntosh CH, Pederson RA (1995) Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 136:3585–3596
Holst JJ, Deacon CF (1998) Inhibition of the activity of dipeptidyl peptidase IV activity as a treatment for type 2 diabetes. Diabetes 47:1663–1670
Adelhorst K, Hedegaard BB, Knudsen LB, Kirk O (1994) Structure-activity studies of glucagon-like peptide-1. J Biol Chem 269:6275–6278
Mentlein R, Gallwitz B, Schmidt WE (1993) Dipeptidyl peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1 (7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem 214:829–835
Balkan B, Kwasnik L, Miserendino R, Holst JJ, Li, X (1999) Inhibition of dipeptidyl peptidase IV with NVP-DPP728 increases plasma GLP-1 (7-36 amide) concentrations and improves oral glucose tolerance in obese Zucker rats. Diabetologia 42:1324–1331
Deacon CF, Hughes TE, Holst JJ (1998) Dipeptidyl peptidase IV inhibition potentiates the insulinotropic effect of glucagon-like peptide-1 in anesthetized pigs. Diabetes 47:764–769
Deacon CF, Wamberg S, Bie P, Hughes TE, Holst JJ (2002) Preservation of active incretin hormones by inhibition of dipeptidyl peptidase IV suppresses meal-induced incretin secretion in dogs. J Endocrinol 172:355–362
Marguet D, Baggio L, Kobayashi T et al (2000) Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci U S A 97:6874–6879
Pederson RA, White HA, Schlenzig D, Pauly RP, McIntosh CH, Demuth HU (1998) Improved glucose tolerance in Zucker fatty rats by oral administration of the dipeptidyl peptidase IV inhibitor isoleucine thiazolidide. Diabetes 47:1253–1258
Pospisilik JA, Stafford SG, Demuth HU et al (2002) Long-term treatment with the dipeptidyl peptidase IV inhibitor P32/98 causes sustained improvements in glucose tolerance, insulin sensitivity, hyperinsulinemia, and beta-cell glucose responsiveness in VDF (fa/fa) Zucker rats. Diabetes 51:943–950
Sudre B, Broqua P, White RB et al (2002) Chronic inhibition of circulating dipeptidyl peptidase IV by FE 999011 delays the occurrence of diabetes in male Zucker diabetic fatty rats. Diabetes 51:1461–1469
Ahrén B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A (2004) Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 89:2078–2084
Ahrén B, Simonsson E, Larsson H et al (2002) Inhibition of dipeptidyl peptidase IV improves metabolic control over a 4-week study period in type 2 diabetes. Diabetes Care 25:869–875
Turner AJ, Isaac RE, Coates D (2001) The neprilysin (NEP) family of zinc metallopeptidases: genomics and function. Bioessays 23:261–269
Hupe-Sodmann K, Göke R, Göke B et al (1997) Endoproteolysis of glucagon-like peptide (GLP)-1 (7-36) amide by ectopeptidases in RINm5F cells. Peptides 18:625–632
Hupe-Sodmann K, McGregor GP, Bridenbaugh R et al (1995) Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1(7-36) amide and comparison of the substrate specificity of the enzyme for other glucagon-like peptides. Regul Pept 58:149–156
Northridge DB, Jardine AG, Alabaster CT et al (1989) Effects of UK 69 578: a novel atriopeptidase inhibitor. Lancet 2:591–593
Kaye B, Brearley CJ, Cussans NJ, Herron M, Humphrey MJ, Mollatt AR (1997) Formation and kinetics of the active drug candoxatrilat in mouse, rabbit, dog and man following administration of the prodrug candoxatril. Xenobiotica 27:1091–1102
Deacon CF, Danielsen P, Klarskov L, Olesen M, Holst JJ (2001) Dipeptidyl peptidase IV inhibition reduces the degradation and clearance of GIP and potentiates its insulinotropic and anti-hyperglycemic effects in anesthetized pigs. Diabetes 50:1588–1597
Trebbien R, Klarskov L, Olesen M, Holst JJ, Carr RD, Deacon CF (2004) Neutral endopeptidase 24.11 is important for the degradation of both endogenous and exogenous glucagon in anesthetized pigs. Am J Physiol Endocrinol Metab 287:E431–E438
Plamboeck A, Holst JJ, Carr RD, Deacon CF (2002) Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both involved in regulating the metabolic stability of glucagon-like peptide-1 in vivo. In: Hildebrandt M, Klapp B, Hoffman T, Demuth HU (eds) Dipeptidyl aminopeptidases in health and disease: advances in experimental medicine and biology, vol 524. Kluwer Academic/Plenum, NY, pp 303–312
Neubert K, Born I, Faust J et al (1983) Verfahren zur Herstellung neuer Inhibitoren der Dipeptidyl Peptidase IV. German Patent Application Number DD 296 075 A5
Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ (1996) Glucagon-like peptide-1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am J Physiol 271:E458–E464
Ørskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ (1994) Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 43:535–539
Ørskov C, Jeppesen J, Madsbad S, Holst JJ (1991) Proglucagon products in the plasma of non-insulin dependent diabetics and nondiabetic controls in the fasting state and following oral glucose and intravenous arginine. J Clin Invest 87:415–423
Agersø H, Jensen LB, Elbrønd B, Rolan P, Zdravkovic M (2002) The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia 45:195–202
Selmeci L, Szokodi I, Horvat-Karajz K (1996) A sensitive microplate-based continuous-monitoring (kinetic) assay for serum neutral endopeptidase (EC 3.4.24.11) activity. Clin Chim Acta 244:111–116
Danilewicz JC, Barclay PL, Barnish IT et al (1989) UK-69,578, a novel inhibitor of EC 3.4.24.11 which increases endogenous ANF levels and is natriuretic and diuretic. Biochem Biophys Res Commun 164:58–65
Roques BP, Noble F, Dauge V, Fournie-Zaluski MC, Beaumont A (1993) Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacol Rev 45:87–146
Northridge DB, Currie PF, Newby DE et al (1999) Placebo-controlled comparison of candoxatril, an orally active neutral endopeptidase inhibitor, and captopril in patients with chronic heart failure. Eur J Heart Fail 1:67–72
Yoshida K, Kanazawa M, Casley DJ, Katopothis A, Johnston CI (1998) Inhibition of kidney neutral endopeptidase after administration of the neutral endopeptidase inhibitor candoxatril: quantitation by autoradiography. J Cardiovasc Pharmacol 32:702–708
Hansen L, Deacon CF, Orskov C, Holst JJ (1999) Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 140:5356–5363
Fulcher IS, Matsas R, Turner AJ, Kenny AJ (1982) Kidney neutral endopeptidase and the hydrolysis of enkephalin by synaptic membranes show similar sensitivity to inhibitors. Biochem J 203:519–522
Chipkin RE, Kreutner W, Billard W (1984) Potentiation of the hypoglycemic effect of insulin by thiorphan, an enkephalinase inhibitor. Eur J Pharmacol 102:151–154
Blais C Jr, Fortin D, Rouleau JL, Molinaro G, Adam A (2000) Protective effect of omapatrilat, a vasopeptidase inhibitor, on the metabolism of bradykinin in normal and failing human hearts. J Pharmacol Exp Ther 295:621–626
Arbin V, Claperon N, Fournié-Zaluski M-C, Roques BP, Peyroux J (2001) Acute effect of the dual angiotensin-converting enzyme and neutral endopeptidase 24-11 inhibitor mixanapril on insulin sensitivity in obese Zucker rat. Br J Pharmacol 133:495–502
Arbin V, Claperon N, Fournié-Zaluski M-C, Roques BP, Peyroux J (2003) Effects of dual angiotensin-converting enzyme and neutral endopeptidase 24-11 chronic inhibition by mixanapril on insulin sensitivity in lean and obese Zucker rats. J Cardiovasc Pharmacol 41:254–264
Russell JC, Kelly SE, Schäfer S (2004) Vasopeptidase inhibition improves insulin sensitivity and endothelial function in the JCR:LA-cp rat. J Cardiovasc Pharmacol 44:258–265
Wang CH, Leung N, Lapointe N et al (2003) Vasopeptidase inhibitor omapatrilat induces profound insulin sensitization and increases myocardial glucose uptake in Zucker fatty rats. Circulation 107:1923–1929
Schäfer S, Linz W, Bube A et al (2003) Vasopeptidase inhibition prevents nephropathy in Zucker diabetic fatty rats. Cardiovasc Res 60:447–454
Lin MC, Wright DE, Hruby VJ, Rodbell M (1975) Structure–function relationships in glucagon: properties of highly purified des-His-1-, monoiodo-, and (des-Asn-28, Thr-29)(homoserine lactone-27)-glucagon. Biochemistry 14:1559–1563
Deacon CF, Ahrén B, Holst JJ (2004) Inhibitors of dipeptidyl peptidase IV: a novel approach for the prevention and treatment of type 2 diabetes? Expert Opin Investig Drugs 13:1091–1102
Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ (2001) Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 50:609–613
Acknowledgements
The excellent technical assistance of S. Pilgaard, M. Olesen and L. Klarskov is gratefully acknowledged. We thank A. Rosendahl and B. Svendsen (Novo Nordisk, Bagsværd, Denmark) for measuring the intact GLP-1 concentrations.These studies were supported by the Danish Medical Research Council, the Novo Nordisk Foundation, the Velux Foundation and the Danish Biotechnology Programme.
Duality of interest R. D. Carr is an employee of, and holds shares in, Novo Nordisk, Bagsværd, Denmark. C. F. Deacon and J. J. Holst have consulted for Novo Nordisk.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plamboeck, A., Holst, J.J., Carr, R.D. et al. Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia 48, 1882–1890 (2005). https://doi.org/10.1007/s00125-005-1847-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-1847-7