Abstract
Aims/hypothesis
Our aim was to compare the therapeutic effect of thalidomide and rosiglitazone on the prevention of diabetic retinopathy in streptozotocin-induced diabetic rats.
Methods
Male Holtzman rats of 6 to 8 weeks of age and weighing 170±30 g were randomly divided into four groups: control (n=13), untreated diabetic (n=17) and diabetic rats treated with thalidomide (200 mg kg–1 day–1) (n=8) or rosiglitazone (1 mg kg–1 day–1) (n=22) for 3 months. Diabetes was induced by streptozotocin with the rats having a body weight of 70 mg/kg. After treatment, vascular endothelial growth factor (VEGF) concentrations in ocular fluid were compared between the different groups, and retinal capillary basement membrane thickness was measured by electron microscopy.
Results
Higher VEGF concentrations in ocular fluid and thicker basement membranes were observed in untreated diabetic rats compared to the control rats. Similar VEGF concentrations and basement membrane thickness were observed for the thalidomide-treated group compared with the control group, whereas no difference in these parameters was observed between the rosiglitazone-treated rats and the control or untreated diabetic rats.
Conclusions/interpretation
Our findings confirm the association between VEGF concentrations and diabetic retinopathy as suggested by other investigators. Thalidomide, but not rosiglitazone, was associated with the inhibition of basement membrane thickening and the blockade of the increase of VEGF in ocular fluid, thus representing a potential therapeutic drug for the prevention of diabetic retinopathy.
Similar content being viewed by others
Diabetes mellitus, one of the major public health problems, is a highly prevalent disease and an important cause of mortality and morbidity because of the chronic complications frequently associated with it [1]. Diabetic retinopathy is the most common and specific vascular complication of both Type 1 and Type 2 diabetes, with 70 to 100% of patients with Type 1 diabetes being estimated to develop retinal alterations that may result in blindness [2, 3]. Although strict glycaemic control has been shown to considerably reduce the incidence of retinopathy, it is not easily achieved and maintained. Its efficacy has not been completely confirmed being that 12% of diabetic patients under intensive treatment develop retinopathy [3, 4].
Proliferative diabetic retinopathy is characterised by the extensive formation of new blood vessels in the retina (angiogenesis) and invasion towards the vitreous body, followed by haemorrhage and the traditional detachment of the retina, which lead to visual alterations and blindness [5, 6]. The presence of ischaemic areas in the retina is fundamental for angiogenic stimulation, which is mediated by growth factors, including VEGF [7, 8, 9, 10, 11, 12, 13, 14, 15]. It is currently believed that retinopathy is an inflammatory process, since various studies have shown that the circulating leukocytes activated in diabetic patients play a crucial role in the increased adhesion of leukocytes, macrophages and vascular adhesion molecules and in the process of vascular occlusion and retinal ischaemia [16, 17, 18, 19]. Several alterations in the retinal capillaries of diabetic patients are observed with electron microscopy: numerous capillaries with open inter-endothelial junctions, increased cytoplasmic vacuolization (enhanced vesicular transport), pericytes presenting degenerative alterations, and capillary BM (lamina) thickening. These can be reproduced in experimental studies [5, 6, 20]. The cause of this thickening, is still unknown [20, 21]. It alters cellular function and/or oxygen diffusion and reduces the contact between pericytes and endothelial cells, contributing to the vascular alterations observed in diabetic patients [20, 22].
Some drugs such as thalidomide and rosiglitazone have shown an inhibitory action on angiogenesis in vivo and in vitro [23, 24, 25, 26], which might represent a potential preventive therapeutic measure for proliferative retinopathy. Thalidomide has been used successfully for various autoimmune and inflammatory diseases [27, 28, 29] characterised by increased concentrations of macrophage-produced TNF-α and VEGF, because it interferes with cellular adhesion molecules, reducing the expression especially of β2-integrin produced by leukocytes, thus inhibiting cell-matrix interactions [23, 30]. The action of Rosiglitazone (R), a peroxisome proliferator-activated receptor γ (PPAR-γ) ligand, a member of a new class of anti-diabetic drugs, has also been related to the inhibition of macrophages and monocytes, as well as to the inhibition of tumour cell proliferation [31, 32]. A recent study [25] showed that PPAR-γ ligands inhibit angiogenesis in vitro and in vivo, with VEGF inhibition being one of the mechanisms proposed. In a more recent study [24] in vitro inhibition of VEGF-induced choroidal angiogenesis by PPAR-γ ligands.
Our objective was to evaluate the therapeutic action potential of T and R as antiangiogenic agents to prevent the development of diabetic retinopathy in rats as evaluated by BM thickening and VEGF concentration in ocular fluid.
Material and methods
A total of 120 male Holtzman rats of 6 to 8 weeks of age and weighing 170±30 g were randomly divided into two groups: a normal control group and a streptozotocin-induced diabetic group (70 mg/kg by a single intraperitoneal injection). The diabetic rats (glycaemia >250 mg/dl) were subdivided into three groups: untreated diabetic rats, diabetic rats treated with T and diabetic rats treated with R. All experimental procedures and animal care followed the ethical guidelines of the Brazilian College of Animal Experimentation.
Procedures
All rats were maintained in individual cages under adequate conditions at an ambient temperature of 21±2°C on a 12-h light/dark cycle, with standard diet and water available ad libitum. Treatment of diabetic rats was initiated after confirmation of the diabetic state (3 days after induction) with either T tablets (100 mg) provided by the Health Secretariat of the Brazilian State of Minas Gerais, or R tablets (4 mg) (GlaxoSmithKline, Rio de Janeiro, Brazil). The tablets were pulverised and the individual dose, 200 and 1 mg/kg for T [23] and R respectively [33], was calculated. The drugs were mixed daily with a pelleted vehicle consisting of casein and starch prepared by the Animal house staff, and the standard diet (commercial ration for small rodents, NUVILAB CR1, Nuvital Nutrientes, Belo Horizonte, Minas Gerais, Brazil) was given only after the vehicle was completely ingested by the animals. The control animals received the same vehicle diet but without the drug. Weight and fasting glycaemia (capillary blood glucose) were measured every 2 weeks throughout the experiment to adjust the dose of the drug according to weight and to evaluate the diabetic state of the rats.
At the end of the 12-week treatment period, the animals were killed by ethyl ether inhalation, and material was collected immediately for biochemical tests (glucose, insulin and VEGF) and morphological analysis of the retina.
Biochemical tests
Capillary blood samples were collected from the caudal vein after a daytime physiological fast to measure glycaemia, using commercial reagent strips (Advantage, Hofman La Roche, Indianapolis, Ind.,USA) based on an enzymatic method (glucose dehydrogenase). Insulin was measured in duplicate with a commercial liquid phase radioimmunoassay kit (Linco Research, Saint Louis, Mo., USA) using 125I-labelled insulin and an antibody specific for rat insulin.
The VEGF was measured in ocular fluid, a mixture of the aqueous and vitreous humour, and immediately after the rats where killed it was removed by puncturing the ocular globe with disposable 1-ml syringes connected to a 13×3.8 mm needle. The material was stored at −70°C until the time for use. This peptide was measured on microplates coated with rat and mouse VEGF-specific polyclonal antibody by an enzyme immunoassay [11, 12] using a commercial kit (Quantikine M. Murine VEGF Immunoassay, R&D System, Minneapolis, Minn, USA). The detection limit of the method was 7.8 pg/ml, with the standard curve being linear up to 500 pg/ml. All measurements were carried out in duplicate. Analysis of intra-assay variability showed a coefficient of variation (CV) of 8.2% (37.7±3.1 pg/ml) for sample A (40 pg/ml) and of 4.7% (144±6.2 pg/ml) for sample B (150 pg/ml). Inter-assay variations showed a CV of 8.4% for sample A (38.3±3.2 pg/ml) and of 6.7% for sample B (138±7.8 pg/ml). Samples were quantified by colorimetric measurement in a microplate reader equipped with a computerised program, considering the standard curve obtained in the same assay.
Morphological analysis of the retina
Retinal capillary BMT was measured by electron microscopic analysis. After puncture, the retina was enucleated and isolated. The ocular globe was removed surgically and sectioned at the level of the equator of the eyeball to isolate the posterior segment where the retina is located. This tissue sample was cut in a trapezoid shape, with the smaller side, i.e. the portion proximal to the optic nerve, consisting of sclera, choroid and retina, which is easily detached from the remaining tissue.
The material was fixed in 3% glutaraldehyde for 24 h, washed in 0.1 mol/l phosphate buffer, pH 7.2, and postfixed in 1% osmium tetroxide. After washing and dehydration in alcohol, the specimens were immersed in Epon resin and acetone and embedded in Epon DMP-30, with the smaller side facing the surface. After polymerization, the blocks were cut into semi-thin sections and the sections stained with 0.5% toluidine blue and sodium borate. Ultrathin sections were obtained and counter-stained with uranyl acetate and lead citrate. All material was prepared at the Electron Microscopy Center of the Federal University of Minas Gerais (CEMEL) and analysed ultrastructurally with a Zeiss EM 10 electron microscope.
About 20 panoramic photomicrographs of the retinal capillaries were randomly selected from each group. Poor quality images were discarded: control group (16 photomicrograph), untreated diabetic rats (n=14), diabetic rats treated with T (n=8), and diabetic rats treated with R (n=20). Each negative was corrected using the respective magnification factor in such a way that the final magnification for analysis was ×30 000.
The morphometric method used implies the three-dimensional interpretation of the capillary which depends on its section plane; thus, analysis of the capillary lumen area might be important for the interpretation of BMT determined for each capillary. We therefore considered the ratio between BMT and capillary lumen area (BM:lumen) as the comparative parameter between groups.
The basement membrane thickness was measured with a pachymeter at 1-cm intervals around the vessel perimeter and is reported in mm with a total of ten measurements for each vessel. The results were converted into nm and corrected for the vessel lumen area. The capillary lumen area was estimated by multiplying the values obtained for the cross-sectional and longitudinal measurements of the extreme points. In the case of vessels with a quite irregular lumen, two measurements were made at points with a larger and smaller diameter, and the means were then multiplied. Values in cm2 were converted to nm2 and corrected for the magnification of the image (×30 000). The BM:lumen ratio (nm–1) was used as the final measurement.
Statistical analyses
Parametric data obtained in both the longitudinal and cross-sectional study were compared by the paired Student’s t test and, when more than two time points were analysed, by repeated measures of ANOVA, followed by the Student-Newman-Keuls test. Non-parametric data were compared by the Wilcoxon test in the longitudinal study and by the Mann-Whitney U test in the cross-sectional study when no more than two time points were analysed. Otherwise, the Friedman test was applied, followed by the Müller-Dunn test as the post test. An alpha risk value equal to or lower than 5% (p<0.05) of committing a type I or 1st species error, and a beta risk equal to or lower than 20% of committing a type II or 2nd species error were considered for analysis. A p value of less than 0.05 was considered statistically significant.
Results
We started the study with 15 rats in the control group and 35 in each diabetic group; i.e. the untreated group, treated with R and treated with T. At the end we had 13, 17, 22 and 15 respectively. However, it was particularly difficult to get sufficient ocular fluid from the T group to measure the VEGF because those animals were extremely dehydrated. This did not occur with the R group, probably because of its liquid retention effect. At the end it was possible to collect enough material from only eight animals.
Animal weight
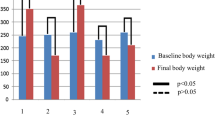
No significant difference in body weight between control and diabetic rats was observed at the beginning of the experiment (170±17 vs 159±25 g, mean±SE, p>0.05). At the end of the 12 weeks, weight gain was higher in the control animals (p<0.001). The final body weight was 268±8 g for the control group, 161.6±3 g for the untreated diabetic group, 152.5±4 g for the T-treated group and 176.6±6 g for the R-treated group. Statistical significance was found just between the final weight in the R-treated group and to the T-treated group (p<0.05, Table 1).
Glucose
Initial glycaemia of the diabetic group was measured 3 days after streptozotocin was injected. The diabetes developed was moderate to severe. Mean glycaemia was higher in the diabetic group than in the control group (p<0.001) (Table 1).
At the end of treatment, no significant difference in glycaemia was observed between untreated, T-treated and R-treated diabetic rats (p>0.05). In the control group, the same concentrations of glycaemia were observed at the beginning and at the end of the 12 weeks.
Insulin
Plasma insulin concentrations were measured at the beginning of the experiment after hyperglycaemia was detected to validate the diabetic model. Control rats showed higher mean insulin concentrations than the diabetic groups (p<0.001).
VEGF in ocular fluid
The VEGF concentration in ocular fluid was higher in untreated diabetic rats than in the control rats (p<0.05) and T-treated diabetic (p<0.01) rats, whereas no significant difference was observed between the untreated diabetic and R-treated rats (p>0.05). There was also no significant difference between the two treated diabetic groups and the control group (p>0.05). On the other hand, VEGF concentrations were lower in the T-treated group compared to the R-treated group (p<0.05, Table 2).
Morphometric study of the basement membrane
Capillary BMT is reported as the mean±SE of individual measurements in each group.
Untreated diabetic rats (n=14) had a thicker BM than the control (n=16) and T-treated rats (n=18) (p<0.001 for both groups), whereas BMT did not differ between untreated and R-treated animals (n=20) (p>0.05). A thicker BM was observed for the R-treated group compared to the control (p<0.001) and T-treated (p<0.01) groups, while there was no difference between control and T-treated rats (p>0.05) (Table 2). Analysis of capillary lumen area showed no significant difference between the four groups (p>0.05, Table 2).
To exclude possible influences of section plane and vessel-diameter, correction of BMT for capillary lumen area (BM/lumen) was done. The results were similar to those obtained before correction, except for the difference observed for the R-treated group, showing a clearly thickened BM compared to the control and T-treated groups, which was no longer significant after correction. These results indicate the absence of any correlation between BMT and capillary lumen in the different groups. Therefore, absolute values of BMT without lumen correction were considered for analysis.
Discussion
This study shows an increase in VEGF concentration in the ocular fluid of untreated diabetic rats compared to control rats, and this increase is associated with retinal capillary BM thickening. These data agree with several studies showing an increase in VEGF in the ocular fluid and serum of diabetic patients before they developed complications of the disease [12, 13, 14, 34].
For long-term studies, the most widely accepted model for the evaluation of retinal complications is the streptozotocin-induced diabetic rat. The retinal lesions observed in rats resemble the initial process of diabetic retinopathy that occurs in humans [20]. Thickening of the BM is always observed in a generalised manner in all species with increased glucose concentrations and represents the first lesion during the retinopathic process which culminates in the formation of new vessels [6, 21]. The mechanisms underlying this thickening are still not well understood, but at least two stages are known to be of fundamental importance in this: (i) increased synthesis of extra-cellular matrix through hyperglycaemia-stimulated biochemical reactions [5, 17, 35], and (ii) reduced proteolysis of laminin and fibronectin (glycoprotein components of the capillary BM) due to an increase in tissue plasminogen activator inhibitor 1 (PAI-1) [35, 36]. The increase in VEGF concentrations has been related to active retinal neovascularization as observed in ischaemic diseases of the retina [11, 14]. Two studies [14, 15] have shown increased VEGF mRNA expression in retinal vessels of diabetic rats, and blockade of the VEGF receptors suppressed capillary BM thickening and retinal neovascularization in other species.
Thus, our data showed that T-treated diabetic rats did not develop the capillary lesions observed in untreated diabetic animals with the same disease duration. The simultaneous presence of normal VEGF concentrations in ocular fluid and the absence of alterations in the capillary BM might suggest an association between them. The VEGF response to the hyperglycaemic stimulus, which is known to stimulate VEGF production in the retina [10, 11, 12, 13], was somehow blocked in T-treated rats. Little is known about the anti-angiogenic properties of T, and no studies have reported that this drug has the ability to inhibit the retinal-capillary BM thickening provoked by diabetic hyperglycaemia. In humans, T is transformed into various metabolites through hepatic metabolization, one of them being responsible for its antiangiogenic as well as teratogenic effects. However, in rats T is not metabolised by the liver and does not show any teratogenic activity [37]. These differences in T metabolism between rats and humans are important when considering that another action of this drug might be responsible for its inhibitory effect of angiogenesis in retinopathy. In contrast, it is known that tissue hypoxia resulting from ischaemic processes is an important stimulus for the production of VEGF [10, 12] and retinal tissue ischaemia caused by capillary occlusion is a physiopathological characteristic of diabetic retinopathy.
Based on the therapeutic efficacy of T in leprosy, AIDS, auto-immune disease and multiple myeloma [28, 38], and based on previous findings on the inhibition of IGF-1-mediated angiogenesis by T in non-diabetic rabbit cornea [23] and on our results, thalidomide could be used for the prevention of diabetic retinopathy. The side effects of this drug do not exclude its therapeutic effects as long as treatment is done properly.
In contrast to the response obtained with the use of T, diabetic rats treated with R showed VEGF concentrations in ocular fluid similar to those observed for untreated diabetic and normal rats but higher than in T-treated diabetic rats. Thus, R was not effective in inhibiting VEGF secretion in hyperglycaemic diabetic rats. In addition, retinal capillary BMT in R-treated rats was similar to that observed for the untreated diabetic group and was significantly greater than in the control and T-treated groups, again indicating an association between VEGF concentrations and BMT. However, when BMT was adjusted for vessel lumen area, which did not differ between groups, no significant difference in the BM:lumen ratio was observed between the R-treated group and the untreated diabetic, T-treated and control groups.
Some authors [39] did not consider this adjustment. This procedure can be important in the assessment of BM thickening if variable dimensions of the capillary BM are observed in the same rat or in the same group of rats due to differences in section planes or vessel size. This situation was not found in the four groups studied, as BMT showed little variation within the same group considering the variations in lumen area of the same capillaries analysed.
Based on the data above, we conclude that R was not effective in preventing the development of capillary lesions in diabetic rats.
The antiangiogenic effect of R was initially shown by [25], who induced angiogenesis in rat cornea with VEGF and observed inhibition of neovascularization in animals treated with PPAR-γ ligands. Another important study [24] also showed the inhibition of choroidal angiogenesis induced by photocoagulation in rats after intravitreous injection of troglitazone. In a more recent study [26] retinal ischaemia was induced in newborn mice by oxygen hyperperfusion and intravitreous injections of R maleate (100 µmol/l) were applied, resulting in inhibition of retinal neovascularization irrespective of VEGF over-expression in the ganglion-cell layer of the retina. This suggests that R inhibited retinal neovascularization by blocking the angiogenic action of VEGF rather than by direct inhibition of this growth factor. Another relevant observation [26] was the inhibition of protein kinase, an enzyme recognised to be important in the development of diabetic retinopathy, in endothelial retinal cells. The same results have been confirmed in in vitro studies [25], differing only in the effect of the dose.
Various studies have shown that the activation of PPAR-γ is associated with endothelial protection [40, 41] and even with the inhibition of monocytes and macrophages [31]. Furthermore, both in vitro and in vivo studies in experimental animals agree that PPAR-γ ligands are inhibitors of angiogenesis, but the exact mechanism remains controversial. Evidence indicates that the effect occurs in the post-VEGF production phase probably by blocking the synthesis of its receptors. These findings are useful for interpreting previous results showing that troglitazone induces an increase in the plasma of VEGF in Type 2 diabetic patients treated with this drug [42].
Our results show that R does not prevent the development of diabetic retinopathy in rats. This is probably related to the inability of R to block the action of VEGF at its receptor level rather than to its inability to reduce the increased VEGF concentrations triggered by the diabetic state. Since the antiangiogenic effect of R is dose-dependent, it is possible that the dose, time and especially the route of administration used in our study were not adequate for this animal model. Therefore oral R at the dose of 1 mg kg–1 day–1 administered for a period of 3 months is probably not effective in preventing retinopathy in streptozotocin-induced diabetic rats.
In conclusion, our data shows increased VEGF concentrations in ocular fluid and retinal capillary-basement-membrane thickening in diabetic rats, compared to normal rats, 3 months after the onset of the disease. Although no differences in the parameters studied were observed after the use of R, daily administration of T to diabetic rats for the same period of 3 months was associated with the blockade of the increase of VEGF in ocular fluid and the inhibition of BM thickening, thus indicating the potential action of this drug in preventing retinal neovascularization in humans (Fig. 1).
Abbreviations
- T:
-
Thalidomide
- R:
-
rosiglitazone
- VEGF:
-
vascular endothelial growth factor
- BM:
-
basement membrane
- BMT:
-
basement membrane thickness
References
Harris MI, Flegal KM, Cowie CC et al. (1998) Prevalence of diabetic, impaired fasting glucose and impaired glucose tolerance in U.S. adults. Diabetes Care 21:518–524
Merimee TJ (1990) Diabetic retinopathy: a synthesis of perspectives. N Engl J Med 322:978–983
Charles MC Jr, Lee DA (1995) Prevention and treatment of the complications of diabetes mellitus. N Engl J Med 332:1210–1217
DCCT Reseach Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent Diabetes Mellitus. N Engl J Med 329:977–986
Imesch PD, Bindley CD, Wallow IHL (1997) Clinicopathologic correlation of intraretinal microvascular abnormalities. Retina 17:321–329
Ishibashi T, Inomato H (1993) Ultrastructure of retinal vessels in diabetic patients. Br J Ophthalmol 77:574–578
Lu M, Kuroki M, Amano S et al. (1998) Advanced glycation and products increase retinal vascular endothelial growth factor expression. J Clin Invest 101:1219–1224
Cavallo MG, Pozzilli P, Bird C (1991) Cytokines in sera from insulin-dependent diabetic patients at diagnosis. Clin Exp Immunol 68:256–259
Murata T, Nakagawa K, Khalil A, Ishibashi I, Inomata H, Sueishi K (1996) The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas. Lab Invest 74:819–825
Paques M, Massin P, Gaudric A (1997) Growth factors and diabetic retinopathy. Diabetes Metab 23:152–60
Frank RN (1994) Vascular endothelial growth factor—its role in retinal vascular proliferation. N Engl J Med 331:1519–1520
Aiello LP, Avery RL, Arrigg PG et al. (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487
Tanaka Y, Katoh S, Hori S, Miura M, Yamashita H (1997) Vascular endothelial growth factor in diabetic retinopathy. Lancet 349:1520–1526
Clermont AC, Aiello LP, Mori F, Aiello LM, Bursell SE (1997) Vascular endothelial growth factor and severity of nonproliferative diabetic retinopathy mediate retinal hemodynamics in vivo: a potential role for vascular endothelial growth factor in the progression of nonproliferative diabetic retinopathy. Am J Ophthalmol 124:433–446
Aiello LP, Pierce EA, Foley ED et al. (1995) Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA 92:10457–10461
Miyamoto K, Khosrol S, Bursell SE et al. (1999) Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci USA 96:10836–10841
Schröder S, Palinski W, Schmid-Schönbein S (1991) Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol 139:81–98
McLeod DS, Lefer DJ, Merges C, Lutty GA (1995) Enhanced expression of intracellular adhesion molecule-1 and P-selection in the diabetic human retina and choroid. Am J Pathol 147:642–653
Barouch FC, Miyamoto K, Allport JR et al. (2000) Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest Ophthalmol Vis Sci 41:1153–1158
Engerman RL, Kern TS (1995) Retinopathy in animal models of diabetes. Diabetes Metab Rev 11:109–120
Wallow IHL, Engerman RL (1977) Permeability and patency of retinal blood vessels in experimental diabetes. Invest Ophthalmol Vis Sci 16:447–461
Hirschi KK, D’Amore PA (1997) Control of angiogenesis by the pericyte: molecular mechanisms and significance. Exs 79:419–428
Amato RJ, Loughnan MS, Flynn E, Folkman J (1994) Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA 91:4082–4085
Murata T, He S, Hangai M et al. (2000) Peroxisome proliferator-activated receptor {gamma} ligands inhibit choroidal neovascularization. Invest Ophthalmol Vis Sci 41:2309–2317
Xin X, Yang S, Kowalski J, Gerritsen ME (1999) Peroxisome proliferator-activated receptor γ ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem 274:9116–9121
Murata T, Hata Y, Ishibashi T, Kim S, Law RE (2001) Response of experimental retinal neovascularization to thiazolidinediones Arch Ophthalmol 119:709–717
Ghigliotti G, Repetto T, Farris A, Roy MT, De Marchi R (1993) Thalidomide: treatment of choice for aphthous ulcers in patients seropositive for human immunodeficiency virus. J Am Acad Dermatol 28:271–272
Raje N, Anderson K (1999) Thalidomide—a revival story. N Engl J Med 341:1606–1609
Oliver SJ, Cheng TP, Banquerigo ML, Brahn E (1998) The effect of thalidomide and 2 analogs on collagen induced arthritis. J Rheumatol 25:964–969
Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G (1991) Thalidomide selectively inhibits tumor necrosis factor alfa production by stimulated human monocytes. J Exp Med 173:699–703
Jiang C, Ting AT, Seed B (1998) PPAR-γ agonists inhibit production of inflammatory cytokines. Nature 301:82–86
Goetze S, Xi XP, Kawano H et al. (1999) PPAR gamma-ligands inhibit migration mediated by multiple chemoattractants in vascular smooth muscle cells. J Cardiovasc Pharmacol 33:798–806
Young PW, Cawthone MA, Coyle PJ et al. (1995) Reapet treatment of obese mice with BRL-49653, a new potent insulin sensitizer, enhances insulin action in white adipocytes. Association with increased insulin binding and cell-surface GLUT4 as measured by photoaffinity labeling. Diabetes 44:1087–1092
Chiarelli F, Spagnoli A, Basciani F et al. (2000) Vascular endothelial growth factor (VEGF) in children, adolescents and young adults with type 1 diabetes mellitus: relation to glycemic control and microvascular complications. Diabetic Med 17:650–656
Spoerri PE, Robison Jr WG, Player D et al. (1998) Diabetes related increase in plasminogen activator inhibitor 1 expression in monkey retinal capillaries. Int Diabetes 6:15–27
Roy S, Sala R, Cagliero E, Lorenzi M (1990) Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci USA 87:404–408
Bauer KS, Dixon SC, Figg WD (1998) Inhibition of angiogenesis by thalidomide requires metabolic activation, which is species-dependent. Biochem Pharmacol 55:1827–1834
Calabrese L, Fleischer AB (2000) Thalidomide: current and potential clinical applications. Am J Med 108:487–495
Grant MB, Spoerri PE, Player DW et al. (2000) Plasminogen activator inhibitor (PAI)-1 overexpression in retinal microvessels of PAI-1 transgenic mice. Invest Ophthalmol Vis Sci 41:2296–2303
Walker AB, Naderali EK, Chattington PD, Buckingham RE, Williams G (1998) Differential vasoactive effects of the insulin sensitizers rosiglitazone (BRL 49653) and troglitazone on human small arteries in vitro. Diabetes 47:810–814
Walker AB, Chattington PD, Buckingham RE, Williams G (1999) The thiazolidinedione rosiglitazone (BRL 49653) lowers blood pressure and protects against impairment of endothelial function in Zucker fatty rats. Diabetes 48:1448–1453
Emoto M, Anno T, Sato Y et al. (2001) Troglitazone treatment increases plasma vascular endothelial growth factor in diabetic patients and its mRNA in 3T3-l1 adipocytes. Diabetes 50:1166–1170
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00125-004-1377-8
Rights and permissions
About this article
Cite this article
Bosco, A.A., Lerario, A.C., Santos, R.F. et al. Effect of thalidomide and rosiglitazone on the prevention of diabetic retinopathy in streptozotocin-induced diabetic rats. Diabetologia 46, 1669–1675 (2003). https://doi.org/10.1007/s00125-003-1234-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-003-1234-1