Abstract
Vernalization-2 (Vrn-2) is the major flowering repressor in temperate cereals. It is only expressed under long days in wild-type plants. We used two day-neutral (photoperiod insensitive) mutations that allow rapid flowering in short or long days to investigate the day length control of Vrn-2. The barley (Hordeum vulgare) early maturity8 (eam8) mutation affects the barley ELF3 gene. eam8 mutants disrupt the circadian clock resulting in elevated expression of Ppd-H1 and the floral activator HvFT1 under short or long days. When eam8 was crossed into a genetic background with a vernalization requirement Vrn-2 was expressed under all photoperiods and the early flowering phenotype was partially repressed in unvernalized (UV) plants, likely due to competition between the constitutively active photoperiod pathway and the repressing effect of Vrn-2. We also investigated the wheat (Triticum aestivum) Ppd-D1a mutation. This differs from eam8 in causing elevated levels of Ppd-1 and TaFT1 expression without affecting the circadian clock. We used genotypes that differed in “short-day vernalization”. Short days were effective in promoting flowering in individuals wild type at Ppd-D1, but not in individuals that carry the Ppd-D1a mutation. The latter showed Vrn-2 expression in short days. In summary, eam8 and Ppd-D1a mimic long days in terms of photoperiod response, causing Vrn-2 to become aberrantly expressed (in short days). As Ppd-D1a does not affect the circadian clock, this also shows that clock regulation of Vrn-2 operates indirectly through one or more downstream genes, one of which may be Ppd-1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The environmental cues of day length (photoperiod) and extended periods of low temperature (vernalization) are used by many plant species to regulate the timing of flowering during the year. Correct timing is an important component of adaptation in crops and has a strong effect on yield (reviewed by Cockram et al. 2007). In the model plant Arabidopsis thaliana (Arabidopsis subsequently) the photoperiod pathway involves circadian regulation of CONSTANS (CO) so that expression peaks approximately 16 h after dawn. Under long-day (LD) conditions the coincidence of light with CO protein allows the induction of FLOWERING LOCUS T (FT) in vascular tissue in leaves (Valverde et al. 2004). FT protein moves via the phloem to the apex where it interacts with the FD protein to activate SOC1 and AP1, leading to floral transition (Abe et al. 2005; Wigge et al. 2005). The principal flowering repressor in Arabidopsis is the MADS-box transcription factor FLOWERING LOCUS C (FLC) that represses FT, FD and SOC1 (reviewed by Higgins et al. 2010). FLC is stably down regulated by epigenetic modification during vernalization (reviewed by Amasino 2010; Crevillén and Dean 2010) allowing inductive pathways, such as photoperiod, to promote flowering.
Well conserved homologues of the core components of the circadian clock and the photoperiod pathway (GI, CO and FT) are found in other plants including cereals, suggesting that the core photoperiod pathway is conserved (reviewed by de Montaigu et al. 2010; Song et al. 2010; Higgins et al. 2010). However, additional genes have been recruited in different lineages. In cereals, examples of the latter are ID1, Ehd7 and Ghd7 in rice (Oryza sativa) (reviewed by Higgins et al. 2010). In temperate cereals such as barley (H. vulgare) and bread or pasta wheat (T. aestivum, T. durum, respectively) the pseudo-response regulator Ppd-1, which has homology to circadian clock genes of Arabidopsis, is an important regulator of flowering (Turner et al. 2005; Beales et al. 2007; Wilhelm et al. 2009; Shaw et al. 2012). FT1, the likely orthologue of FT in Arabidopsis, can be regulated by Ppd-1 independently of CO (Campoli et al. 2012; Shaw et al. 2012).
In contrast to photoperiod, vernalization pathways show much greater divergence (reviewed by Distelfeld et al. 2009a; Greenup et al. 2009). No homologue of the Arabidopsis FLC gene has been identified in cereals. Instead the role of flowering repressor is taken by VERNALIZATION-2 (Vrn-2), a member of a grass-specific subgroup of CCT domain genes, a family that also includes CO and Ppd-1 (Yan et al. 2004; Mizuno and Nakamichi 2005; Higgins et al. 2010). Loss of function mutations of Vrn-2 allow flowering without vernalization (Yan et al. 2004; Distelfeld et al. 2009b).
Two other genes involved in vernalization have been identified in cereals. Vrn-1 is a MADS-box transcription factor related to the AP1/FUL subgroup in Arabidopsis (Yan et al. 2003; reviewed by Higgins et al. 2010). Vrn-1 expression increases gradually during vernalization (Danyluk et al. 2003; Murai et al. 2003; Yan et al. 2003). Vrn-3 has proved to be the same gene as FT1 (Yan et al. 2006; reviewed by Higgins et al. 2010) and is called FT1 in this paper. Winter cereals, which require vernalization, have wild-type alleles at all three loci. Spring cereals, which have no, or reduced, vernalization requirement, have a mutation of one or more of the Vrn genes.
Current models for temperate cereals are that VRN-2 protein represses FT1 (VRN-3) to prevent flowering, as proposed by Yan et al. (2006). Hemming et al. (2008) demonstrated that overexpression of HvVrn2 down-regulates HvFT1 and represses flowering. Vrn-1 is induced during vernalization and represses Vrn-2, allowing FT1 expression to be induced by LDs (Trevaskis et al. 2006; Hemming et al. 2008; Distelfeld et al. 2009b; Chen and Dubcovsky 2012). Several lines of evidence have indicated that photoperiod, and photoperiod sensitivity, play a role in the activity of Vrn-2. Karsai et al. (2005) found that the presence/absence of HvVrn2 only affected vernalization response in LD; there was no response in SD. In addition, Hemming et al. (2008) found that rapid flowering of plants carrying deletions at HvVrn2 was dependent on the presence of an active Ppd-H1 allele.
Vrn-2 is expressed principally in leaves of wheat and barley (Yan et al. 2004; Sasani et al. 2009) under LD but not short-day (SD) conditions (Dubcovsky et al. 2006; Trevaskis et al. 2006). This contrasts with Arabidopsis where the expression of FLC is not dependent on day length (Sung et al., 2006). These findings suggest that Vrn-2 acts as a LD repressor, preventing flowering in winter cereals germinating in summer or autumn when day lengths would be sufficient to activate photoperiod response. This is similar to the LD repression role of the related CCT domain gene Ghd7 in rice (Xue et al. 2008).
Wild-type barley and wheat are LD plants, flowering earlier under LDs than SDs (photoperiod responsive or photoperiod sensitive). However, both species have day-neutral (or photoperiod insensitive) mutations that enable rapid flowering under SD or LD conditions. The control of Vrn-2 expression by photoperiod and the availability of different day-neutral mutations provided a novel opportunity to investigate the interaction between photoperiod and vernalization. In barley we used an early maturity8 (eam8) mutation that affects an orthologue of the Arabidopsis circadian clock gene EARLY FLOWERING3 (ELF3) gene (Faure et al. 2012; Zakhrabekova et al. 2012). eam8 affects the expression of genes that are components of the circadian clock (HvCCA1, HvTOC1 and HvGI) and causes constitutive activation of photoperiod response with high levels of Ppd-H1 and HvFT1 expression. This leads to rapid flowering under SD or LD conditions (Faure et al. 2012). eam8 mutant stocks are in spring barley varieties that do not require vernalization because of mutations in Vrn-1 and Vrn-2. For our experiments we crossed eam8 into a winter barley background with functional Vrn-1 and Vrn-2 alleles.
In wheat we used the Ppd-D1a mutation. This has a promoter deletion associated with constitutive expression. In contrast to eam8 the Ppd-D1a mutation does not affect the expression of genes in the circadian clock (TaCCA1, TaTOC1 or TaGI), but does affect downstream photoperiod pathway components resulting in induction of TaFT1 expression under SD conditions (Beales et al. 2007; Wilhelm et al. 2009; Shaw et al. 2012). We also utilised the observation that SDs can partially substitute for cold treatment in some wheat varieties, a phenomenon known as “short-day vernalization” (Purvis and Gregory 1937; Roberts et al. 1988) or “short-day induction” (Evans 1987). We investigated Vrn-2 expression in wild-type, eam8 and Ppd-D1a mutant genotypes and related this to flowering time in vernalized (V) and UV plants. This enabled us to study the interaction between photoperiod and vernalization pathways and to determine whether variation in short-day vernalization behaviour was attributable to allelic variation at Ppd-1.
Materials and methods
Plant material
Development of the ‘Igri (eam8)’ lines is described in Faure et al. (2012). Seeds of wheat varieties were obtained from the Genetic Resources Unit of the John Innes Centre.
Growth conditions for barley
Seeds were germinated to coleoptile lengths of 1–3 cm and V for 6 weeks at 4 °C in 8 h days. Ten days before the end of vernalization additional seeds were germinated and V and UV plants were grown together in SD (9 h light, 15 h dark) or LD (16 h light, 8 h dark) conditions (136 μmol m−2 s−1 light; 16 °C). Ten plants of each genotype were grown for each treatment combination. Flowering time was recorded as the date when awns had emerged 2 cm from the flag leaf.
For quantitative RT-PCR plants were grown as above and entire above-ground material from seedlings were sampled into liquid nitrogen on the days and at the time points described in “Results”. Each sample comprised material from three seedlings, and three samples were taken per treatment. Error bars on graphs show the standard error of the mean (n = 3).
For the continuous light experiment UV ‘Igri’ seedlings were grown in a 12 h light/12 h dark cycle (136 μmol m−2 s−1 light; 16 °C) for 16 days and then given continuous light for the remainder of the experiment. Three samples of above-ground material (biological replicates) were taken into liquid nitrogen every 4 h with each sample comprising material from three seedlings.
Growth conditions for wheat
Seeds were germinated to coleoptile lengths of 1–3 cm and grown for 4 or 8 weeks in SD (9 h light, 15 h dark; 136 μmol m−2 s−1 light; 16 °C). Leaf tissue (20 mm2) of each individual was taken after 10 days for DNA extraction and PpdD1 allele typing as in Beales et al. (2007).
For quantitative PCR, at 19 days post-germination four individuals from each parental line (‘Maris Templar’, ‘Cappelle-Desprez’ and ‘Krasnodar39’) and four individuals from each category (parental homozygous and heterozygous) from the ‘Cappelle-Desprez’ × ‘Krasnodar39’ cross were selected at random and assayed for Vrn-2 expression were sampled for genotyping and Vrn-2 expression. The results presented in Fig. 6 are therefore the mean and standard deviation of four observations per sample. After the SD treatment, the plants were moved to a glasshouse with natural LDs (>14 h light) and flowering time was recorded as the date of ear emergence (when the spike was 50 % emerged from the flag leaf).
Quantitative RT-PCR
RNA was extracted, cDNA was synthesised and samples were processed essentially as described in Shaw et al. (2012). Tri-Reagent (Sigma) was used for RNA extraction and MMLV-RTase (Invitrogen) with Oligo-dT (12–18) for reverse-transcription. Quantification was achieved using either an Opticon Real-Time PCR instrument (Bio-Rad) or a Roche LightCycler 480 instrument. SybrGreen Fluorescence chemistry was used to quantify amplification. Cycle threshold values were converted into relative mRNA abundance using the ΔC t method using the reaction efficiency values calculated by the instrumentation software. The abundance calculations were performed as \( E^{{ - \Updelta C_{\text{t}} }} \) where E is the reaction efficiency and then normalised by division with the normalising factor, calculated in the same way either singly (18s rRNA, wheat experiments and barley entrainment experiment) or as a geometric mean (18s rRNA and actin mRNA, barley 24 h experiments; Vandesompele et al. 2002) to control for fluctuations in cDNA synthesis efficiency. PCR primers used are listed in Online Resource 1.
Results
Phenotypes of barley eam8 mutant plants
Development of eam8 lines is described in Faure et al. (2012), but briefly the eam8 (ea8.k allele) mutation was crossed to the winter barley ‘Igri’ after which self-pollinated plants were V and selected for early flowering in SDs. From these, plants were selected that were homozygous for the ‘Igri’ alleles at Vrn1 and Vrn2 (determining vernalization requirement) and the photoperiod-sensitive Ppd-H1 ‘Igri’ allele using a combination of PCR-based markers (Turner et al. 2005; Yan et al. 2004; Fu et al. 2005) and phenotype screens. These plants are referred to as ‘Igri(eam8)’.
Flowering time (days to awn emergence on the main stem) was recorded for V and UV plants in controlled environment cabinets with SD (9 h light) or LD (LD; 16 h light) conditions (Fig. 1). For the purposes of this report the term “unvernalized” (UV) refers to plants which have not been subjected to vernalization treatment.
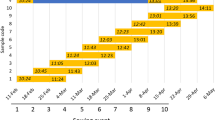
Days to awn emergence of barley cultivars and introgression lines. Vernalized (V) and unvernalized (UV) plants were grown under short days (SD) and long days (LD). Error bars indicate standard error of the mean. Flowering times of vernalized plants of ‘Igri’, ‘Igri(eam8)’ and the eam8 parent were previously published in Faure et al. (2012)
The parental lines behaved as expected from previous studies and their known Ppd and Vrn genotypes. Flowering in ‘Igri’ (winter barley parent) was delayed by at least 80 days in UV plants (LDs or SDs) and in V plants in SDs. The eam8 parent was a spring barley and flowered at similar times in SDs and LDs with or without vernalization, as expected. Additional controls were ‘Triumph’, which carries the ppd-H1 mutation, and ‘Triumph(Ppd-H1)’ which is an introgression line carrying the active Ppd-H1 allele from ‘Igri’ (Turner et al. 2005). ‘Triumph’ (spring growth habit; VrnA1c; Casas et al. 2008) flowered earlier in LDs than SDs and this difference was enhanced by the Ppd-H1 allele. Neither genotype was affected by vernalization, consistent with previous results.
The flowering repressor Vrn-2 is expressed under LDs but not SDs in wild-type plants (Dubcovsky et al. 2006; Trevaskis et al. 2006). This suggested that UV ‘Igri(eam8)’plants would be early flowering under SDs (where VRN-2 is predicted to be absent), and late flowering under LDs (where VRN-2 is normally expressed). However, UV ‘Igri(eam8)’ plants flowered at the same time in SD or LD conditions and were approximately 50 days later than V plants (Fig. 1). This suggested that Vrn-2 was expressed under SD or LD conditions in UV eam8 plants to partially suppress the early flowering phenotype.
Expression of Vrn-2 in wild type and eam8 mutant barley plants
In UV ‘Igri’ plants Vrn-2 was expressed under LDs but was practically undetectable under SDs, consistent with previous studies (Dubcovsky et al. 2006; Trevaskis et al. 2006). Vrn-2 was expressed in UV ‘Igri(eam8)’ plants under SD or LD conditions. Expression was significantly higher than wild type under SDs (Fig. 2). Previous results from a microarray experiment showed that the eam mutant constitutively behaved as if under LDs (Faure et al. 2012). Figure 2 shows that this behaviour extends to the expression of Vrn-2, which was not represented on the array. Expression was reduced after vernalization in ‘Igri’ plants as expected. A fall in the average level was also seen in ‘Igri(eam8)’ plants, especially under LDs, showing that eam8 still responds to vernalisation.
Vrn-2 expression 8 h after dawn in ‘Igri’ and ‘Igri(eam8’) plants comparing vernalized (V) or unvernalized (UV) plants grown under short days (SD) or long days (LD). Error bars indicate standard error of the mean, and columns bearing the same lettering are not significantly different from each other at the 95 % confidence level (one-way ANOVA)
Vrn-2 is a member of the CCT domain family of genes. Other members of this family are known to be components of, or regulated by, the circadian clock (Mizuno and Nakamichi 2005). Vrn-2 has previously been shown to have a diurnal change in expression when measured together with a closely related CCT gene (HvZCCTa and HvZCCTb in Trevaskis et al. 2006). These data suggested that Vrn-2 might have circadian control in wild-type plants. To test this we entrained UV ‘Igri’ plants in 12 h light/12 h dark conditions for 16 days and then moved them to continuous light and measured expression at 3 h intervals over 48 h. Vrn-2 expression was very low in the 12 h/12 h conditions, but rose rapidly 16 h after transfer to continuous light. Expression then fell during the subjective night and rose again at 56 h (Fig. 3). This showed circadian control of Vrn-2 in wild-type plants and also showed that expression was strongly induced by the first exposure to 16 h or more of light. The plants sampled for expression in Fig. 2 were harvested at the start of the day which explains the relatively low level of Vrn-2 expression in wild-type plants.
Expression of Ppd-H1, HvCO1, HvFT1 and Vrn-1 in wild type and eam8 mutant barley plants
To investigate the effect of vernalization further, expression of Ppd-H1, HvCO1, HvFT1 and Vrn-1 were compared in V and UV seedlings of ‘Igri’ and ‘Igri(eam8)’ grown under SD and LD conditions and sampled at subjective dawn (Fig. 4). This time point was selected because expression levels were previously found to be low in ‘Igri’ and high in ‘Igri(eam8)’ using V plants under SDs (Faure et al. 2012). Plants in the present study behaved similarly. PpdH1 expression was almost undetectable in ‘Igri’ wild-type plants, but was expressed under all conditions in ‘Igri(eam8)’ plants (Fig. 4a). Neither changes in day length or vernalization caused consistent changes in PpdH1 expression in ‘Igri(eam8)’ plants. Greater expression was seen in V plants in SD than LD, and vice versa for UV plants; vernalization produced an increase in expression in SD plants but a non-significant reduction in LD plants. There was a tendency for HvCO1 expression to be increased in SD relative to LD for ‘Igri(eam8)’ plants (Fig. 4b), while the reverse was seen in ‘Igri’ plants, but vernalization had no effect on HvCO1 expression, which was always low in ‘Igri’ and high in ‘Igri(eam8)’. In contrast, vernalization has a strong effect on HvFT1 expression (Fig. 4c), which was greatly reduced in UV plants of both genotypes, most likely due to high levels of Vrn-2. The deregulation of expression of Vrn-2 seen in ‘Igri(eam8)’ plants was not observed for Vrn-1 (Fig. 4d), where expression was almost undetectable in UV plants whether or not the eam8 allele was present. In V plants the eam8 allele was associated with increased expression of Vrn-1.
Expression of photoperiod pathway genes at dawn in ‘Igri’ and ‘Igri(eam8)’ plants comparing vernalized (V) or unvernalized (UV) grown under short days (SD) or long days (LD). Error bars indicate standard error of the mean, and columns bearing the same lettering are not significantly different from each other at the 95 % confidence level (one-way ANOVA). a Ppd-H1, b HvCO1, c HvFT1, d Vrn-1
These results show that eam8 plants lost the normal day length control of Vrn-2, expressing the gene under SD and LD conditions (Fig. 2), consistent with the delayed flowering phenotype under both conditions. One explanation is that Vrn-2 is directly controlled by the circadian clock which is disrupted in the eam8 mutant (Faure et al. 2012). An alternative possibility is that Vrn-2 is controlled by genes regulated by the clock but downstream of the clock itself. For example, Ppd-H1 expression is deregulated and increased in eam8 plants. To test the control of Vrn-2 further, we examined wheat plants carrying the dominant Ppd-D1a mutation. This causes over expression of Ppd-D1 and a day-neutral early flowering phenotype, but does not affect the expression of the circadian clock genes TaCCA1, TaTOC1 or TaGI (Shaw et al. 2012).
Expression of Vrn-2 in wild type and photoperiod-insensitive (Ppd) mutant wheat plants
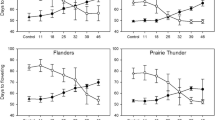
To study Vrn-2 expression in wheat, we utilised a known observation that wheat varieties differ in their interaction between day length and vernalization. Some winter (vernalization requiring) varieties of cereals are able to flower after a period of growth in SDs at non-vernalizing temperatures, a phenomenon known as “short-day vernalization” (Purvis and Gregory 1937; Roberts et al. 1988). In diploid wheat (T. monococcum) this has been shown to be associated with loss of Vrn-2 expression (Dubcovsky et al. 2006). It had also been noted that varieties which failed to respond to short-day vernalization were often known to carry the photoperiod-insensitive (day neutral) mutation Ppd1. For this paper we reinvestigated hexaploid winter wheat varieties previously studied by Davidson et al. (1985). In Davidson et al.’s experiments different lengths of vernalization treatment were followed by growth at non-vernalizing temperatures in SD or LD conditions. They showed that the English variety ‘Maris Templar’ flowered after approximately 125 days in SDs irrespective of the vernalization treatment while in LDs flowering was strongly delayed in plants given less than 4 weeks vernalization and occurred most rapidly in plants that were fully V (Fig. 5a). Thus, ‘Maris Templar’ exhibited “short-day vernalization”. In contrast, the Russian variety ‘Krasnodar 39’ did not, and behaved similarly when grown under SD or LD conditions, with flowering always delayed by no or shorter vernalization times (Fig. 5a).
Days to flowering in wheat varieties differing in short-day vernalization response. a Days to flowering of wheat cultivars ‘Maris Templar’ (MT) and ‘Krasnodar 39’ (Kr) subjected to different vernalization periods then grown under continuous short days (SD) or long days (LD). Redrawn from Figure 3 of Davidson et al. (1985). b Days to ear emergence of wheat cultivars ‘Maris Templar’, ‘Cappelle-Desprez’ and ‘Krasnodar 39’ after 4 or 8 weeks vernalization (‘vern’) or an equivalent period in short days (‘SD’) and a non-vernalizing temperature of 16 °C before transfer to a glasshouse under natural warm LD conditions
We used a different protocol to confirm the observation that SDs could substitute for vernalization. We grew plants of ‘Maris Templar’, ‘Cappelle-Desprez’ (also studied by Davidson et al. (1985) and showed to behave like ‘Maris Templar’) and ‘Krasnodar 39’ for 0, 4 or 8 weeks at a non-vernalizing temperature of 16 °C in SDs (9 h light) before transferring plants to a glasshouse under natural warm LD conditions. Flowering times (days to ear emergence after transfer to the glasshouse; Fig. 5b) showed that the SD treatment could substitute for equivalent periods of vernalization in ‘Maris Templar’ and ‘Cappelle-Desprez’ in that plants reached ear emergence at equivalent dates (N.B. at transfer the SD grown plants were considerably larger and more developed). The 4 or 8 week SD treatment was much less effective in ‘Krasnodar 39’ (Fig. 5b), consistent with the inability of this variety to respond to short-day vernalization found by Davidson et al. (1985).
Genotyping using assays described in Beales et al. (2007) showed that ‘Maris Templar’ and ‘Cappelle-Desprez’ were photoperiod-sensitive varieties (wild-type Ppd-1 alleles) while ‘Krasnodar 39’ carried the photoperiod-insensitive (day neutral) Ppd-D1a mutation. To determine if this explained the difference in short-day vernalization response, we first assayed Vrn-2 expression in UV plants. Whilst all three varieties showed similar levels of Vrn-2 expression under LD (data not shown), only ‘Krasnodar 39’ expressed Vrn-2 strongly under SD conditions (Fig. 6a).
Vrn-2 expression and flowering times in wheat. a Vrn-1 and Vrn-2 expression in wheat seedlings grown for 18 days in SD. Left panel shows expression in seedlings of cultivars ‘Maris Templar’, ‘Cappelle-Desprez’ and ‘Krasnodar 39’, and right panel shows expression in individuals of a ‘Cappelle-Desprez’ × ‘Krasnodar 39’ F2 population when classified according to Ppd-D1 allele status. Error bars indicate standard error of the mean. b Days to ear emergence of a ‘Cappelle-Desprez’ × ‘Krasnodar 39’ F2 population subjected to 4 weeks in SD then moved to natural LD. Individuals are classified according to Ppd-D1 allele status. Open squares are PpdD1b individuals, black squares are PpdD1a individuals, and grey squares are heterozygotes
We then examined the relationship between Ppd-D1, SD expression of Vrn-2, and SD-vernalization competence in a segregating population. F2 seedlings from a ‘Cappelle-Desprez’ × ‘Krasnodar 39’ cross were grown initially at 16 °C in SDs (9 h light). Plants were genotyped for Ppd-D1 and at 19 days post-germination four individuals from each category (parental homozygous and heterozygous) were selected at random and assayed for Vrn-2 expression. Plants homozygous for the wild-type (photoperiod sensitive) Ppd-D1b allele had very low levels of Vrn-2 expression while expression was high in heterozygotes and in plants homozygous for the dominant day-neutral Ppd-D1a allele (Fig. 6b). After 4 weeks the plants were moved to natural LDs in a glasshouse and days to ear emergence were recorded. Photoperiod-sensitive Ppd-D1b homozygote individuals flowered ahead of plants carrying the mutant Ppd-D1a allele (Fig. 6c), showing that variation in SD-vernalization in these genotypes could be explained by allelic variation at Ppd-1 and associated variation in Vrn-2 expression. Ppd-D1a, like eam8, therefore causes plants to behave as if under LDs in terms of photoperiod response and Vrn-2 expression.
Discussion
The flowering repressor Vrn-2 was previously shown to be expressed under LD but not SD conditions in wild-type plants (Dubcovsky et al. 2006; Trevaskis et al. 2006). It was also shown to have diurnal variation in expression in LDs (Trevaskis et al. 2006). In this paper, we show that Vrn-2 is regulated by the circadian clock. Use of two contrasting mutations that cause early flowering under SD or LD allowed the regulation of Vrn-2 and the interaction of photoperiod and vernalization to be explored in more detail.
In terms of photoperiod response the day-neutral eam8 mutation of barley and the Ppd-D1a mutation of wheat cause the plants to behave as if they are under LDs, irrespective of the actual day length. A result of this is that plants with either mutation express Vrn-2 under SD or LD conditions. ELF3 is an important component of the circadian clock in Arabidopsis (McWatters et al. 2000) with elf3 mutants showing clock arrhythmia under constant conditions (Hicks et al. 2001; Thines and Harmon 2010). EAM8 is a barley orthologue of ELF3 and the early flowering eam8 phenotype results from loss of gene function (Faure et al. 2012; Zakhrabekova et al. 2012). eam8 mutants have altered expression of circadian clock genes (HvCCA1, HvTOC1 and HvGI) and downstream components of the photoperiod pathway so that Ppd-H1 expression is increased and HvFT1 is expressed under SD or LD conditions (Faure et al. 2012). The Ppd-D1a mutant of wheat has no effect on the circadian clock, at least in normal light/dark cycles, but causes constitutive expression of the Ppd-D1 gene itself and expression of TaFT1 under SDs (Shaw et al. 2012).
Our data show there are at least two routes to Vrn-2 expression in SD: via clock disruption and associated activation of the photoperiod pathway, as in ‘Igri(eam8)’; and by activation of the photoperiod pathway without clock disruption using the PpdD1a allele. From this we conclude that Vrn-2 is not directly regulated by the clock, but is instead under the control of one or more clock-regulated downstream components. Although Ppd-1 itself is a candidate it should be noted that Ppd-1 is expressed under SDs in wild-type plants even though Vrn-2 is not. Therefore, it would be the altered timing of Ppd-1 expression during the day that is significant in the mutants. This model predicts that a reduction in Ppd-H1 activity would reduce Vrn-2 expression. The recessive ppd-H1 mutation in barley provides an appropriate test as this is a loss or reduced function allele that delays flowering in LDs but has no effect in SDs (Turner et al. 2005). However, Hemming et al. (2008) found that Vrn-2 levels in LDs did not differ between Ppd-H1 and ppd-H1 plants. Therefore, the mechanism by which the day-neutral mutations affect Vrn-2 expression remains unclear and requires further study.
It has been proposed that Vrn-2 affects flowering by repressing FT1 (Vrn-3) expression (Hemming et al. 2008; Chen and Dubcovsky 2012). The observation in this paper that vernalization did not repress two candidate upstream regulators of FT1 (Ppd-H1 and HvCO1) is consistent with this, supporting that idea that vernalization and photoperiod pathways converge on FT and its orthologues in Arabidopsis and cereals, despite the fact that different vernalization genes are involved (FLC in Arabidopsis and Vrn-1/Vrn-2 in cereals). From these results we explain the observed effects of vernalization in this paper as follows. In barley, flowering in UV ‘Igri(eam8)’ plants under SDs or LDs was repressed because Vrn-2 was expressed under both conditions (Fig. 2). Flowering occurred significantly earlier than in the UV ‘Igri’ parent, suggesting a quantitative competition between the promoting effect of the photoperiod pathway, which is strongly enhanced in eam8 plants, and the repressive effect of the vernalization pathway. This conclusion is also supported by the genetic interactions between Vrn-1, Vrn-2 and Ppd-H1 described by Hemming et al. (2008). In wheat we interpret flowering times and the phenomenon of “short-day vernalization” in Fig. 5a as follows: in photoperiod-sensitive winter wheat varieties such as ‘Maris Templar’ and ‘Cappelle-Desprez’ the flowering time in SDs is consistent irrespective of the vernalization treatment because there is no flowering repression (Vrn-2 is not expressed in SDs) and no photoperiodic promotion. The fact that flowering occurs in SDs implies the existence of an additional pathway which could involve ambient temperature or age. FT3 (related to FT1) may be significant as in barley it is gradually upregulated in SDs (Faure et al. 2007). It seems likely that HvFT3, like HvFT1, is involved in the integration of signals from the vernalization and photoperiod pathways, as its expression is affected by both photoperiod (Faure et al. 2007) and by the requirement for a functional copy of Vrn-2. Casao et al. (2011) found that barley plants with the spring growth habit caused by Vrn-2 deletions expressed HvFT3 at high levels in the absence of vernalization, and that this deregulation allowed expression in normally repressive LD conditions, leading them to propose Vrn-2 as a repressor of HvFT3.
In LDs there is high Vrn-2 expression in plants that are not V, and this suppresses flowering. As vernalization proceeds, Vrn-2 gradually becomes repressed and the LD photoperiod response is increasingly able to promote flowering. In ‘Krasnodar 39’ the Ppd-D1a mutation causes the plants to behave as if they are under LDs, irrespective of the actual day length, so that Vrn-2 is expressed under SD or LD conditions. Consequently, SD and LD flowering times were similar for each vernalization time (Fig. 5a). Flowering in LD was always slightly earlier than in SD, possibly because of an additional promoting effect from the wild-type Ppd alleles on the other genomes. In our experiments (Fig. 5b), growth in SDs for 4 or 8 weeks was sufficient to commit wild-type plants to flowering, again because there was no Vrn-2 mediated repression. SDs were less efficient for ‘Krasnodar 39’ because Vrn-2 was expressed due to the presence of the day-neutral Ppd-D1a mutation.
Work in rice has shown that the Ghd7 gene, which is a repressor of flowering in LDs, is a member of the same grass-specific subgroup of CCT domain genes as Vrn-2 (Xue et al. 2008; reviewed by Higgins et al. 2010). This suggests that LD suppression was an ancestral property of cereals and that the evolution of temperate cereals involved at least two key changes. One was a change to LD promotion for the photoperiod pathway. The second was the evolution of a vernalization pathway involving Vrn-1 being able to repress Vrn-2 after a period of low temperature. These changes enabled ancestral cereals to adopt a winter annual life style with suppression of flowering in late summer and autumn and rapid flowering under increasing day lengths in the following spring.
References
Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309:1052–1056
Amasino R (2010) Seasonal and developmental timing of flowering. Plant J 61:1001–1013
Beales J, Turner A, Griffiths S, Snape JW, Laurie DA (2007) A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor Appl Genet 115:721–733
Campoli C, Drosse B, Searle I, Coupland G, von Korff M (2012) Functional characterisation of HvCO1, the barley (Hordeum vulgare) flowering time ortholog of CONSTANS. Plant J 69:868–880
Casao MC, Igartua E, Karsai I, Lasa JM, Gracia MP, Casas AM (2011) Expression analysis of vernalization and day-length response genes in barley (Hordeum vulgare L.) indicates that VRNH2 is a repressor of PPDH2 (HvFT3) under long days. J Exp Bot 62:1939–1949
Casas AM, Yahiaoui S, Cuesta A, Ciudad FJ, Molina-Cano JL, Karsai I, Meszaros K, Lasa JM, Gracia MP, Hayes PM, Igartua E, Szucs P (2008) Vrn-H1 and Vrn-H2 allelic diversity in barley may explain specific adaptation to the Mediterranean environments. Options Mediterraneennes. Serie A, Seminaires Mediterraneens, pp 105–109
Chen A, Dubcovsky J (2012) Wheat TILLING mutants show that the vernalization gene VRN1 down-regulates the flowering repressor VRN2 in leaves but is not essential for flowering. PLoS Genet. doi:10.1371/journal.pgen.1003134
Cockram J, Jones H, Leigh FJ, O’Sullivan D, Powell W et al (2007) Control of flowering time in temperate cereals: genes, domestication, and sustainable productivity. J Exp Bot 58:1231–1244
Crevillén P, Dean C (2010) Regulation of the floral repressor gene FLC: the complexity of transcription in a chromatin context. Curr Opin Plant Biol 14:38–44
Danyluk J, Kane NA, Breton G, Limin AE, Fowler DB, Sarhan F (2003) TaVRT-1, a putative transcription factor associated with vegetative to reproductive transition in cereals. Plant Physiol 132:1849–1860
Davidson JL, Christian KR, Jones DB, Bremner PM (1985) Responses of wheat to vernalization and photoperiod. Aust J Agric Res 36:347–359
de Montaigu A, Réka Tóth R, Coupland G (2010) Plant development goes like clockwork. Trends Genet 26:296–306
Distelfeld A, Li C, Dubcovsky J (2009a) Regulation of flowering in temperate cereals. Curr Opin Plant Biol 12:1–7
Distelfeld A, Tranquilli G, Li C, Yan L, Dubcovsky J (2009b) Genetic and molecular characterization of the VRN2 loci in tetraploid wheat. Plant Phys 149:245–257
Dubcovsky J, Loukoianov A, Fu D, Valarik M, Sanchez A, Yan Y (2006) Effect of photoperiod on the regulation of wheat vernalization genes VRN1 and VRN2. Plant Mol Biol 60:469–480
Evans LT (1987) Short day induction of inflorescence initiation in some winter wheat varieties. Aust J Plant Physiol 14:277–286
Faure S, Higgins J, Turner A, Laurie DA (2007) The flowering locus T-like gene family in barley (Hordeum vulgare). Genetics 176:599–609
Faure S, Turner AS, Gruszka D, Christodoulou V, Davis SJ, von Korff M, Laurie DA (2012) Mutation at the circadian clock gene EARLY MATURITY 8 adapts domesticated barley (Hordeum vulgare) to short growing seasons. Proc Natl Acad Sci USA 109(21):8328–8333
Fu D, Szucs P, Yan L, Helguera M, Skinner JS, von Zitzewitz J, Hayes PM, Dubcovsky J (2005) Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol Genet Genom 273:54–65
Greenup A, Peacock WJ, Dennis ES, Trevaskis B (2009) The molecular biology of seasonal flowering-responses in Arabidopsis and the cereals. Ann Bot 103:1165–1172
Hemming MN, Peacock WJ, Dennis ES, Trevaskis B (2008) Low-temperature and day length cues are integrated to regulate flowering locus T in barley. Plant Physiol 147:355–366
Hicks KA, Albertson TM, Wagner DR (2001) EARLY FLOWERING3 encodes a novel protein that regulates circadian clock function and flowering in Arabidopsis. Plant Cell 13:1281–1292
Higgins JA, Bailey PC, Laurie DA (2010) Comparative genomics of flowering time pathways using Brachypodium distachyon as a model for the temperate grasses. PLoS ONE 5(4):e10065
Karsai I, Szucs P, Meszaros K, Filichkina T, Hayes PM, Skinner JS, Lang L, Bedo Z (2005) The Vrn-H2 locus is a major determinant of flowering time in a facultative × winter growth habit barley (Hordeum vulgare L.) mapping population. Theor Appl Genet 110(8):1458–1466
McWatters HG, Bastow RM, Hall A, Millar AJ (2000) The ELF3 zeitnehmer regulates light signalling to the circadian clock. Nature 408:716–720
Mizuno T, Nakamichi N (2005) Pseudo-response regulators (PRRs) or true oscillator components (TOCs). Plant Cell Physiol 46:677
Murai K, Miyamae M, Kato H, Takumi S, Ogihara Y (2003) WAP1, a wheat APETALA1 homolog, plays a central role in the phase transition from vegetative to reproductive growth. Plant Cell Physiol 44:1255–1265
Purvis ON, Gregory FG (1937) Studies in vernalization of cereals. I. A comparative study of vernalization of winter rye by low temperature and short days. Ann Bot 1:569–592
Roberts EH, Summerfield RJ, Cooper JP, Ellis RH (1988) Environmental control of flowering in barley (Hordeum vulgare L.). I. Photoperiod limits to long-day responses, photoperiod-insensitive phases and effects of low-temperature and short-day vernalization. Ann Bot 62:127–144
Sasani S, Hemming MN, Oliver SN, Greenup A, Tavakkol-Afshari R, Mahfoozi S, Poustini K, Sharifi H-R, Dennis ES, Peacock WJ et al (2009) The influence of vernalization and day length on expression of flowering-time genes in the shoot apex and leaves of barley (Hordeum vulgare). J Exp Bot 60:2169–2178
Shaw LM, Turner AS, Laurie DA (2012) The impact of photoperiod insensitive Ppd-1a mutations on the photoperiod pathway across the three genomes of hexaploid wheat (Triticum aestivum). Plant J 71:71–84
Song YH, Ito S, Imaizumi T (2010) Similarities in the circadian clock and photoperiodism in plants. Curr Opin Plant Biol 13:1–10
Sung S, Schmitz RJ, Amasino RM (2006) A PHD finger protein involved in both the vernalization and photoperiod pathways in Arabidopsis. Genes Dev 20:3244–3248
Thines B, Harmon FG (2010) Ambient temperature response establishes ELF3 as a required component of the core Arabidopsis circadian clock. Proc Natl Acad Sci USA 107:3257–3262
Trevaskis B, Hemming MN, Peacock WJ, Dennis ES (2006) HvVRN2 responds to day length, whereas HvVRN1 is regulated by vernalization and developmental status. Plant Physiol 140:1397–1405
Turner A, Beales J, Faure S, Dunford RP, Laurie DA (2005) The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310:1031–1034
Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G (2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303:1003–1006
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3(7):research0034.1. (Ahead of epub)
Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D (2005) Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309:1056–1059
Wilhelm EP, Turner AS, Laurie DA (2009) Photoperiod insensitive Ppd-A1a mutations in tetraploid wheat (Triticum durum Desf.). Theoret Appl Genet 118:285–294
Xue WY, Xing YZ, Weng XY, Zhao Y, Tang WJ, Wang L, Zhou HJ, Yu SB, Xu CG, Li XH, Zhang QF (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40:761–767
Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J (2003) Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci USA 100:6263–6268
Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J (2004) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303:1640–1644
Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J (2006) The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Natl Acad Sci USA 103:19581–19586
Zakhrabekova S, Gough SP, Braumann I, Müller AH, Lundqvist J, Ahmann K, Dockter C, Matyszczak I, Kurowska M, Druka A, Waugh R, Graner A, Stein N, Steuernageld B, Lundqvist U, Hansson M (2012) Induced mutations in circadian clock regulator Mat-a facilitated short-season adaptation and range extension in cultivated barley. Proc Natl Acad Sci USA 109:4326–4331
Acknowledgments
This study was supported by United Kingdom Biotechnology and Biological Sciences Research Council (BBSRC) Grant 208/D19952 (to S.F., A.S.T., and D.A.L.) and BBSRC Grant-in-Aid (to S.F., A.S.T. and D.A.L., and the John Innes Centre). Y.Z. was supported by a Rotation Studentship from the John Innes Foundation.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The research described herein complies with UK Law.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by X. Xia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Turner, A.S., Faure, S., Zhang, Y. et al. The effect of day-neutral mutations in barley and wheat on the interaction between photoperiod and vernalization. Theor Appl Genet 126, 2267–2277 (2013). https://doi.org/10.1007/s00122-013-2133-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-013-2133-6