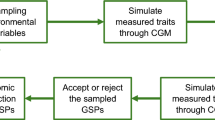
Abstract
The genetic determinism of developmental stages in grapevine was studied in the progeny of a cross between grapevine cultivars Riesling and Gewurztraminer by combining ecophysiological modelling, genetic analysis and data mining of the grapevine whole genome sequence. The dates of three phenological stages, budbreak, flowering and veraison, were recorded during four successive years for 120 genotypes in the vineyard. The phenotypic data analysed were the duration of three periods expressed in thermal time (degree-days): 15 February to budbreak (Bud), budbreak to flowering (Flo) and flowering to veraison (Ver). Parental and consensus genetic maps were built using 153 microsatellite markers on 188 individuals. Six independent quantitative trait loci (QTLs) were detected for the three phases. They were located on chromosomes 4 and 19 for Bud, chromosomes 7 and 14 for Flo and chromosomes 16 and 18 for Ver. Interactions were detected between loci and also between alleles at the same locus. Using the available grapevine whole-genome sequences, candidate genes underlying the QTLs were identified. VvFT, on chromosome 7, and a CONSTANS-like gene, on chromosome 14, were found to colocalise with the QTLs for flowering time. Genes related to the abscisic acid response and to sugar metabolism were detected within the confidence intervals of QTLs for veraison time. Their possible roles in the developmental process are discussed. These results raise new hypotheses for a better understanding of the physiological processes governing grapevine phenology and provide a framework for breeding new varieties adapted to the future predicted climatic conditions.




Similar content being viewed by others
References
Almada R, Cabrera N, Casaretto JA, Ruiz-Lara S, Villanueva EG (2009) VvCO and VvCOL1, two CONSTANS homologous genes, are regulated during flower induction and dormancy in grapevine buds. Plant Cell Rep 28:1193–1203
Amasino RM, Michaels SD (2010) The timing of flowering. Plant Physiol 154:516–520
Baggiolini M (1952) Les stades repères dans le développement annuel de la vigne et leur utilisation pratique. Rev Rom Agric Vitic 8:4–6
Battilana J, Costantini L, Emanuelli F, Sevini F, Segala C, Moser S, Velasco R, Versini G, Grando MS (2009) The 1-deoxy-d-xylulose 5-phosphate synthase gene co-localizes with a major QTL affecting monoterpene content in grapevine. Theor Appl Genet 118:653–669
Bellin D, Peressotti E, Merdinoglu D, Wiedemann-Merdinoglu S, Adam-Blondon AF, Cipriani G, Morgante M, Testolin R, Di Gaspero G (2009) Resistance to Plasmopara viticola in grapevine ‘Bianca’ is controlled by a major dominant gene causing localised necrosis at the infection site. Theor Appl Genet 120:163–176
Blasi P, Blanc S, Wiedemann-Merdinoglu S, Prado E, Rühl E, Mestre P, Merdinoglu D (2011) Construction of a reference linkage map of Vitis amurensis and genetic mapping of Rpv8 a locus conferring resistance to grapevine downy mildew. Theor Appl Genet 123:43–53
Bottcher C, Keyzers RA, Boss PK, Davies C (2010) Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3-1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. J Exp Bot 61:3615–3625
Broman KW, Wu H, Sen S, Churchill GA (2003) R/qtl: QTL mapping in experimental crosses. Bioinformatics 19:889–890
Cakir B, Agasse A, Gaillard C, Saumonneau A, Delrot S, Atanassova R (2003) A grape ASR protein involved in sugar and abscisic acid signaling. Plant Cell 15:2165–2180
Calonje M, Cubas P, Martinez-Zapater JM, Carmona MJ (2004) Floral meristem identity genes are expressed during tendril development in grapevine. Plant Physiol 135:1491–1501
Carmona MJ, Calonje M, Martinez-Zapater JM (2007) The FT/TFL1 gene family in grapevine. Plant Mol Biol 63:637–650
Chervin C, Deluc L (2010) Ethylene signalling receptors and transcription factors over the grape berry development: gene expression profiling. Vitis 49:129–136
Chervin C, Tira-umphon A, Terrier N, Zouine M, Severac D, Roustan JP (2008) Stimulation of the grape berry expansion by ethylene and effects on related gene transcripts, over the ripening phase. Physiol Plant 134:534–546
Costantini L, Battilana J, Lamaj F, Fanizza G, Grando MS (2008) Berry and phenology-related traits in grapevine (Vitis vinifera L.): from quantitative trait loci to underlying genes. BMC Plant Biol 8:38
Decroocq V, Fave MG, Hagen L, Bordenave L, Decroocq S (2003) Development and transferability of apricot and grape EST microsatellite markers across taxa. Theor Appl Genet 106:912–922
Di Gaspero G, Cattonaro F (2010) Application of genomics to grapevine improvement. Aust J Grape Wine Res 16:122–130
Diaz-Riquelme J, Lijavetzky D, Martinez-Zapater JM, Carmona MJ (2009) Genome-wide analysis of MIKCC-Type MADS Box genes in grapevine. Plant Physiol 149:354–369
Doligez A, Adam-Blondon AF, Cipriani G, Di Gaspero G, Laucou V, Merdinoglu D, Meredith CP, Riaz S, Roux C, This P (2006) An integrated SSR map of grapevine based on five mapping populations. Theor Appl Genet 113:369–382
Duchêne E, Schneider C (2005) Grapevine and climatic changes: a glance at the situation in Alsace. Agron Sustain Dev 25:93–99
Duchêne E, Butterlin G, Claudel P, Dumas V, Jaegli N, Merdinoglu D (2009) A grapevine (Vitis vinifera L.) deoxy-d-xylulose synthase gene colocates with a major quantitative trait loci for terpenol content. Theor Appl Genet 118:541–552
Duchêne E, Huard F, Dumas V, Schneider C, Merdinoglu D (2010) The challenge of adapting grapevine varieties to climate change. Clim Res 41:193–204
Gambetta GA, Matthews MA, Shaghasi TH, McElrone AJ, Castellarin SD (2010) Sugar and abscisic acid signaling orthologs are activated at the onset of ripening in grape. Planta 232:219–234
Garcia de Cortazar Atauri I, Brisson N, Gaudillere JP (2009) Performance of several models for predicting budburst date of grapevine (Vitis vinifera L.). Int J Biometeorol 53:317–326
Herrmann D, Barre P, Santoni S, Julier B (2010) Association of a CONSTANS-LIKE gene to flowering and height in autotetraploid alfalfa. Theor Appl Genet 121:865–876
Hey SJ, Byrne E, Halford NG (2010) The interface between metabolic and stress signalling. Ann Bot 105:197–203
Hoth S, Niedermeier M, Feuerstein A, Hornig J, Sauer N (2010) An ABA-responsive element in the AtSUC1 promoter is involved in the regulation of AtSUC1 expression. Planta 232:911–923
Huang T, Bohlenius H, Eriksson S, Parcy F, Nilsson O (2005) The mRNA of the Arabidopsis gene FT moves from leaf to shoot apex and induces flowering. Science 309:1694–1696
Jaillon O, Aury JM, Noel B, Policriti A, Clepet C, Casagrande A, Choisne N, Aubourg S, Vitulo N, Jubin C, Vezzi A, Legeai F, Hugueney P, Dasilva C, Horner D, Mica E, Jublot D, Poulain J, Bruyere C, Billault A, Segurens B, Gouyvenoux M, Ugarte E, Cattonaro F, Anthouard V, Vico V, Del Fabbro C, Alaux M, Di Gaspero G, Dumas V, Felice N, Paillard S, Juman I, Moroldo M, Scalabrin S, Canaguier A, Le Clainche I, Malacrida G, Durand E, Pesole G, Laucou V, Chatelet P, Merdinoglu D, Delledonne M, Pezzotti M, Lecharny A, Scarpelli C, Artiguenave F, Pe ME, Valle G, Morgante M, Caboche M, Adam-Blondon AF, Weissenbach J, Quetier F, Wincker P (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449:U463–U465
Jiang WB, Yu DQ (2009) Arabidopsis WRKY2 transcription factor mediates seed germination and postgermination arrest of development by abscisic acid. BMC Plant Biol 9:96
Jimenez-Gomez JM, Alonso-Blanco C, Borja A, Anastasio G, Angosto T, Lozano R, Martinez-Zapater JM (2007) Quantitative genetic analysis of flowering time in tomato. Genome 50:303–315
Jones GV, White MA, Cooper OR, Storchmann K (2005) Climate change and global wine quality. Clim Change 73:319–343
Keilin T, Pang X, Venkateswari J, Halaly T, Crane O, Keren A, Ogrodovitch A, Ophir R, Volpin H, Galbraith D, Or E (2007) Digital expression profiling of a grape-bud EST collection leads to new insight into molecular events during grape-bud dormancy release. Plant Sci 173:446–457
Kline KG, Sussman MR, Jones AM (2010) Abscisic acid receptors. Plant Physiol 154:479–482
Koyama K, Sadamatsu K, Goto-Yamamoto N (2010) Abscisic acid stimulated ripening and gene expression in berry skins of the Cabernet Sauvignon grape. Funct Integr Genomic 10:367–381
Lee JH, Park SH, Lee JS, Ahn JH (2007a) A conserved role of SHORT VEGETATIVE PHASE (SVP) in controlling flowering time of Brassica plants. BBA-Gene Regul Mech 1769:455–461
Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH (2007b) Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Gene Dev 21:397–402
Marguerit E, Boury C, Manicki A, Donnart M, Butterlin G, Nemorin A, Wiedemann-Merdinoglu S, Merdinoglu D, Ollat N, Decroocq S (2009) Genetic dissection of sex determinism, inflorescence morphology and downy mildew resistance in grapevine. Theor Appl Genet 118:1261–1278
Martinez-Zapater JM, Carmona MJ, Diaz-Riquelme J, Fernandez L, Lijavetzky D (2010) Grapevine genetics after the genome sequence: challenges and limitations. Aust J Grape Wine Res 16:33–46
Mathiason K, He D, Grimplet J, Venkateswari J, Galbraith D, Or E, Fennell A (2009) Transcript profiling in Vitis riparia during chilling requirement fulfillment reveals coordination of gene expression patterns with optimized bud break. Funct Integr Genomic 9:81–96
Mazzitelli L, Hancock RD, Haupt S, Walker PG, Pont SDA, McNicol J, Cardle L, Morris J, Viola R, Brennan R, Hedley PE, Taylor MA (2007) Co-ordinated gene expression during phases of dormancy release in raspberry (Rubus idaeus L.) buds. J Exp Bot 58:1035–1045
Melzer R, Wang YQ, Theissen G (2010) The naked and the dead: the ABCs of gymnosperm reproduction and the origin of the angiosperm flower. Semin Cell Dev Biol 21:118–128
Merdinoglu D, Butterlin G, Bevilacqua L, Chiquet V, Adam-Blondon AF, Decroocq S (2005) Development and characterization of a large set of microsatellite markers in grapevine (Vitis vinifera L.) suitable for multiplex PCR. Mol Breed 15:349–366
Nakagawa H, Yamagishi J, Miyamoto N, Motoyama M, Yano M, Nemoto K (2005) Flowering response of rice to photoperiod and temperature: a QTL analysis using a phenological model. Theor Appl Genet 110:778–786
Olsson A, Engström P, Söderman E (2004) The homeobox genes ATHB12 and ATHB7 encode potential regulators of growth in response to water deficit in Arabidopsis. Plant Mol Biol 55:663–677
Ophir R, Pang XQ, Halaly T, Venkateswari J, Lavee S, Galbraith D, Or E (2009) Gene-expression profiling of grape bud response to two alternative dormancy-release stimuli expose possible links between impaired mitochondrial activity, hypoxia, ethylene-ABA interplay and cell enlargement. Plant Mol Biol 71:403–423
Owen SJ, Lafond MD, Bowen P, Bogdanoff C, Usher K, Abrams SR (2009) Profiles of abscisic acid and its catabolites in developing Merlot grape (Vitis vinifera) berries. Am J Enol Vitic 60:277–284
Pacey-Miller T, Scott K, Ablett E, Tingey S, Ching A, Henry R (2003) Genes associated with the end of dormancy in grapes. Funct Integr Genomic 3:144–152
Pierre J-B, Bogard M, Herrmann D, Huyghe C, Julier B (2010) A CONSTANS-like gene candidate that could explain most of the genetic variation for flowering date in Medicago truncatula. Mol Breed 28:25–35
Quilot B, Kervella J, Génard M, Lescourret F (2005) Analysing the genetic control of peach fruit quality through an ecophysiological model combined with a QTL approach. J Exp Bot 422:3083–3092
Ramos MC, Jones GV, Martinez-Casasnovas JA (2008) Structure and trends in climate parameters affecting winegrape production in northeast Spain. Clim Res 38:1–15
Reymond M, Muller B, Leonardi A, Charcosset A, Tardieu F (2003) Combining quantitative trait loci analysis and an ecophysiological model to analyse the genetic variability of the responses of maize leaf growth to temperatures and water deficit. Plant Physiol 131:664–675
Soar CJ, Sadras VO, Petrie PR (2008) Climate drivers of red wine quality in four contrasting Australian wine regions. Aust J Grape Wine Res 14:78–90
Sreekantan L, Thomas MR (2006) VvFT and VvMADS8, the grapevine homologues of the floral integrators FT and SOC1, have unique expression patterns in grapevine and hasten flowering in Arabidopsis. Funct Plant Biol 33:1129–1139
Sreekantan L, Mathiason K, Grimplet J, Schlauch K, Dickerson JA, Fennell AY (2010) Differential floral development and gene expression in grapevines during long and short photoperiods suggests a role for floral genes in dormancy transitioning. Plant Mol Biol 73:191–205
Uptmoor R, Osei-Kwarteng M, Gurtler S, Stutzel H (2009) Modeling the effects of drought stress on leaf development in a Brassica oleracea doubled haploid population using two-phase linear functions. J Am Soc Hortic Sci 134:543–552
Velasco R, Zharkikh A, Troggio M, Cartwright DA, Cestaro A, Pruss D, Pindo M, Fitzgerald LM, Vezzulli S, Reid J, Malacarne G, Iliev D, Coppola G, Wardell B, Micheletti D, Macalma T, Facci M, Mitchell JT, Perazzolli M, Eldredge G, Gatto P, Oyzerski R, Moretto M, Gutin N, Stefanini M, Chen Y, Segala C, Davenport C, Dematte L, Mraz A, Battilana J, Stormo K, Costa F, Tao Q, Si-Ammour A, Harkins T, Lackey A, Perbost C, Taillon B, Stella A, Solovyev V, Fawcett JA, Sterck L, Vandepoele K, Grando SM, Toppo S, Moser C, Lanchbury J, Bogden R, Skolnick M, Sgaramella V, Bhatnagar SK, Fontana P, Gutin A, Van de Peer Y, Salamini F, Viola R (2007) A high quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS ONE 2:e1326
Walton EF, Wu RM, Richardson AC, Davy M, Hellens RP, Thodey K, Janssen BJ, Gleave AP, Rae GM, Wood M, Schaffer RJ (2009) A rapid transcriptional activation is induced by the dormancy-breaking chemical hydrogen cyanamide in kiwifruit (Actinidia deliciosa) buds. J Exp Bot 60:3835–3848
Wheeler S, Loveys B, Ford C, Davies C (2009) The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Aust J Grape Wine Res 15:195–204
Yin X, Struik PC, van Eeuwijk FA, Stam P, Tang J (2005) QTL analysis and QTL-based prediction of flowering phenology in recombinant inbred lines of barley. J Exp Bot 56:967–976
Acknowledgments
This work was partially financed by the ERA-NET Plant Genomics Program (GRASP GRAPE WINE 072b).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gebhardt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duchêne, E., Butterlin, G., Dumas, V. et al. Towards the adaptation of grapevine varieties to climate change: QTLs and candidate genes for developmental stages. Theor Appl Genet 124, 623–635 (2012). https://doi.org/10.1007/s00122-011-1734-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-011-1734-1