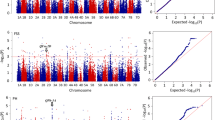
Abstract
There is increasing awareness that epistasis plays a role for the determination of complex traits. This study employed an association mapping approach in a large panel of 455 diverse European elite soft winter wheat lines. The genotypes were evaluated in multi-environment trials and fingerprinted with SSR markers to dissect the underlying genetic architecture of grain yield and heading time. A linear mixed model was applied to assess marker-trait associations incorporating information of covariance among relatives. Our findings indicate that main effects dominate the control of grain yield in wheat. In contrast, the genetic architecture underlying heading time is controlled by main and epistatic effects. Consequently, for heading time it is important to consider epistatic effects towards an increased selection gain in marker-assisted breeding.




Similar content being viewed by others
References
Allison DB, Fernandez JR, Moonseong H, Shankuan Z, Etzel C, Beasley TM, Amos CI (2002) Bias in estimates of quantitative-trait-locus effect in genome scans: demonstration of the phenomenon and a method-of-moments procedure for reducing bias. Am J Hum Genet 70:575–585
Barton NH, Charlesworth B (1998) Why sex and recombination? Science 281:1986–1990
Beavis WB (1998) QTL analyses: power, precision, and accuracy. In: Patterson AH (ed) Molecular dissection of complex traits. CRC Press, Boca Raton
Bernardo R (1993) Estimation of coefficient of coancestry using molecular markers in maize. Theor Appl Genet 85:1055–1062
Boone C, Bussey H, Andrews BJ (2007) Exploring genetic interactions and networks with yeast. Nat Rev Genet 8:437–449
Breseghello F, Sorrells ME (2006) Association mapping of kernel size and milling quality in wheat (Triticum aestivum L.) cultivars. Genetics 172:1165–1177
Buckler ES, Holland JB, Bradbury PJ, Acharya CB, Brown PJ, Browne C, Ersoz E, Flint-Garcia S et al (2009) The genetic architecture of maize flowering time. Science 325:714–718
Campbell BT, Baenzigar PS, Gill KS, Eskridge KM, Budak H, Erayman M, Dweikat I, Yen Y (2003) Identification of QTLs and environmental interactions associated with agronomic traits on chromosome 3A of wheat. Crop Sci 43:1493–1505
Carlborg Ö, Jacobsson L, Ahgren P, Siegel P, Andersson L (2006) Epistasis and the release of genetic variation during long-term selection. Nat Genet 38:418–420
Carver BF, Rayburn AL (1994) Comparison of related wheat stocks possessing 1B or 1RS.1BL chromosomes: agronomic performance. Crop Sci 34:1505–1510
Chao S, Zhang W, Dubcosky J, Sorrels ME (2007) Evaluation of genetic diversity and genome-wide linkage disequilibrium among US wheat (Triticum aestivum L.) germplasm representing different market classes. Crop Sci 47:1018–1030
Cheverud JM, Routman EJ (1996) Epistasis as a source of increased additive genetic variance at population bottlenecks. Evolution 50:1042–1051
Cochran WG, Cox GM (1957) Experimental designs, 2nd edn. John Wiley & Sons, New York
Coyne JA (1992) Genetics and speciation. Nature 355:511–515
Crossa J, Burgueño J, Dreisigacker S, Vargas M, Herrera-Foessel SA, Morten L, Singh RP, Trethowan R, Warburton M, Franco J, Reynolds M, Crouch JH, Ortiz R (2007) Association analysis of historical bread wheat germplasm using additive genetic covariance of relatives and population structure. Genetics 177:1889–1913
Distelfeld A, LI C, Dubcovsky J (2009) Regulation of flowering in temperate cereals. Curr Opin Plant Biol 12:178–184
El-Lithy ME, Bentsink L, Hanhart CJ, Ruys GJ, Rovito D, Broekhof JLM, van der Poel HJA, van Eijk MJT, Vreugdenhil D, Koornneef M (2006) New Arabidopsis recombinant inbred line populations genotyped using SNPWave and their use for mapping flowering-time quantitative trait loci. Genetics 172:1867–1876
Flint-Garcia SA, Thornsberry JM, Buckler ES (2003) Structure of linkage disequilibrium in plants. Annu Rev Plant Biol 54:357–374
Gilmour AR, Gogel BJ, Cullis BR, Thompson R (2006) ASReml User Guide Release 2.0. VSN International, Hemel Hempstead
Goldringer I, Brabant P, Gallais A (1997) Estimation of additive and epistatic genetic variances for agronomic traits in a population of doubled-haploid lines of wheat. Heredity 79:60–71
Goodnight CJ (1987) On the effect of founder events on epistatic genetic variance. Evolution 41:80–91
Göring HHH, Terwilliger JD, Blangero J (2001) Large upward bias in estimation of locus-specific effects from genome-wide scans. Am J Hum Genet 69:1357–1369
Gower JC (1966) Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 53:325–338
Griffiths S, Simmonds J, Leverington M, Wang Y, Fish L, Sayers L, Alibert L, Orford S, Wingen L, Herry L, Faure S, Laurie D, Bilham L, Snape J (2009) Meta-QTL analysis of the genetic control of ear emergence in elite European winter wheat germplasm. Theor Appl Genet 119:383–395
Habier D, Totir LR, Fernando RL (2010) A Two-stage approximation for analysis of mixture genetic models in large pedigrees. Genetics 185:655–670
Hack H, Bleiholder H, Buhr L, Meier U, Schnock-Fricke U, Weber E, Witzenberger A (1992) Einheitliche Codierung der phänologischen Entwicklungsstadien mono- und dikotyler Pflanzen–Erweiterte BBCH-Skala, Allgemein. Nachrichtenbl Deut Pflanzenschutzd 44:265–270
Hallauer AR, Miranda JB (1981) Quantitative genetics in maize breeding. Iowa State University Press, Ames
Hanocq E, Niarquin M, Heumez E, Rousset M, Legouis J (2004) Detection and mapping of QTL for earliness components in a bread wheat recombinant inbred lines population. Theor Appl Genet 110:106–115
Hanocq E, Laperche A, Jaminon O, Lainé AL, Legouis J (2007) Most significant genome regions involved in the control of earliness traits in bread wheat, as revealed by QTL meta-analysis. Theor Appl Genet 114:569–584
Hedrick PW (1987) Gametic disequilibrium measures: proceed with caution. Genetics 117:331–341
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Kearsey MJ, Jinks JL (1968) A general method of detecting additive, dominance and epistatic variation for metrical traits. I. Theory. Heredity 23:403–409
Komeda Y (2004) Genetic regulation of time to flower in Arabidopsis thaliana. Annu Rev Plant Biol 55:521–535
Kraakman ATW, Niks RE, Van den Berg PMMM, Stam P, Van Eeuwijk FA (2004) Linkage disequilibrium mapping of yield and yield stability in modern spring barley cultivars. Genetics 168:435–446
Kuchel H, Hollamby GJ, Langridge P, Williams KJ, Jefferies SP (2006) Identification of genetic loci associated with ear-emergence in bread wheat. Theor Appl Genet 113:1103–1112
Kuchel H, Williams KJ, Langridge P, Eagles HA, Jefferies SP (2007) Genetic dissection of grain yield in bread wheat. I. QTL analysis. Theor Appl Genet 115:1029–1041
Kumar N, Kulwal PL, Balyan HS, Gupta PK (2007) QTL mapping for yield and yield contributing traits in two mapping populations of bread wheat. Mol Breed 19:163–177
Lamkey KR, Schnicker BJ, Melchinger AE (1995) Epistasis in an elite maize hybrid and choice of generation for inbred line development. Crop Sci 35:1272–1281
Law CN, Worland AJ (1997) Genetic analysis of some flowering time and adaptive traits in wheat. New Phytol 137:19–28
Le Rouzic A, Alvarez-Castro JM (2008) Estimation of genetic effects and genotype-phenotype maps. Evol Bioinform 4:225–235
Leamy LJ, Workman MS, Routman EJ, Cheverud JM (2005) An epistatic genetic basis for fluctuating asymmetry of tooth size and shape in mice. Heredity 94:316–325
Li Z, Pinson SRM, Park WD, Paterson AH, Stansel JW (1997) Epistasis for three grain yield components in rice (Oryza sativa L.). Genetics 145:452–465
Li S, Jia J, Wei X, Zhang X, Li L, Chen H, Fan Y, Sun H, Zhao X, Lei T, Xu Y, Jiang F, Wang H, Li L (2007) A intervarietal genetic map and QTL analysis for yield traits in wheat. Mol Breed 20:167–178
Maurer HP, Melchinger AE, Frisch M (2008) Population genetic simulation and data analysis with Plabsoft. Euphytica 161:133–139
McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JPA, Hirschhorn JN (2008) Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet 9:356–369
Melchinger AE, Utz HF, Schön CC (1998) Quantitative trait locus (QTL) mapping using different testers and independent population samples in maize reveals low power of QTL detection and larger bias in estimates of QTL effects. Genetics 149:383–403
Melchinger AE, Piepho H-P, Utz HF, Muninovic J, Wegenast T, Törjek O, Altmann T, Kusterer B (2007) Genetic basis of heterosis for growth-related traits in Arabidopsis investigated by testcross progenies of near-isogenic lines reveals a significant role of epistasis. Genetics 177:1827–1837
Montooth KL, Marden JH, Clark AG (2003) Mapping determinants of variation in energy metabolism, respiration and flight in drosophila. Genetics 165:623–635
Myles S, Peiffer J, Brown PJ, Ersoz ES, Zhang Z, Costich DE, Buckler ES (2009) Association mapping: critical considerations shift from genotyping to experimental design. Plant Cell 21:2194–2202
Nyholt DR, LaForge KS, Kallela M, Alakurtti K, Anttila V, Färkkilä M, Hämaläinen E, Kaprio J, Kaunisto MA et al (2008) A high-density association screen of 155 ion transport genes for involvement with common migraine. Hum Mol Genet 17:3318–3331
Phillips PC (2008) Epistasis–the essential role of gene interactions in the structure and evolution of genetic systems. Nat Rev Genet 9:855–867
Quarrie SA, Steed A, Calestani C, Semikhodskii A, Lebreton C, Chinoy C, Steele N, Pljevljakusic D, Waterman E, Weyen J, Schondelmaier J, Habash DZ, Farmer P, Saker L, Clarkson DT, Abugalieva A, Yessimbekova M, Turuspekov Y, Abugalieva S, Tuberosa R, Sanguineti M-C, Hollington PA, Aragues R, Royo A, Dodig D (2005) A high density genetic map of hexaploid wheat (Triticum aestivum L.) from the cross Chinese Spring × SQ1 and its use to compare QTLs for grain yield across a range of environments. Theor Appl Genet 110:865–880
Quarrie SA, Pekic Quarrie S, Radosevic R, Rancic D, Kaminska A, Barnes JD, Leverington M, Ceoloni C, Dodig D (2006) Dissecting a wheat QTL for yield present in a range of environments: from the QTL to candidate genes. J Exp Bot 57:2627–2637
Reif JC, Hallauer AR, Melchinger AE (2005) Heterosis and heterotic patterns in maize. Maydica 50:215–223
Scarth R, Law CN (1984) The control of the day-length response in wheat by the group 2 chromosomes. Z. Pflanzenzuechtung 92:140–150
Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114
Sourdille P, Snape JW, Cadalen T, Charmet G, Nakata N, Bernard S, Bernard M (2000) Detection of QTLs for heading time and photoperiod response in wheat using a doubled-haploid population. Genome 43:487–494
Stich B, Melchinger AE, Frisch M, Maurer HP, Heckenberger M, Reif JC (2005) Linkage disequilibrium in European elite maize germplasm investigated with SSRs. Theor Appl Genet 111:723–730
Stich B, Melchinger AE, Piepho HP, Hamrit S, Schipprack W, Maurer HP, Reif JC (2007) Potential causes of linkage disequilibrium in a European maize breeding program investigated with computer simulations. Theor Appl Genet 115:529–536
Stich B, Melchinger AE, Heckenberger M, Möhring J, Schechert A, Piepho H-P (2008a) Association mapping in multiple segregating populations of sugar beet (Beta vulgaris L.). Theor Appl Genet 117:1167–1179
Stich B, Möhring J, Piepho H-P, Heckenberger M, Buckler ES, Melchinger AE (2008b) Comparison of mixed-model approaches for association mapping. Genetics 178:1745–1754
Trethowan R, Reynolds MP, Ortiz-Monasterio I, Ortiz R (2007) The genetic basis of the green revolution in wheat production. Plant Breed Rev 28:39–58
Utz HF, Melchinger AE, Schön CC (2000) Bias and sampling error of the estimated proportion of genotypic variance explained by quantitative trait loci determined from experimental data in maize using cross validation and validation with independent samples. Genetics 154:1839–1849
Uwatoko N, Onishi A, Ikeda Y, Kontani M, Sasaki A, Matsubara K, Itoh Y, Sano Y (2008) Epistasis among the three major flowering time genes in rice: coordinate changes of photoperiod sensitivity, basic vegetative growth and optimum photoperiod. Euphytica 163:167–175
Worland AJ, Börner A, Korzun V, Li WM, Petrovic S, Sayers EJ (1998) The influence of photoperiod genes to the adaptability of European winter wheats. Euphytica 100:385–394
Wright S (1978) Evolution and genetics of populations, variability within and among natural populations, 4th edn. The University of Chicago Press, Chicago, p 91
Würschum T, Maurer HP, Schulz B, Möhring J, Reif JC (2011) Genome-wide association mapping reveals epistasis and genetic interaction networks in sugar beet. Theor Appl Genet (in press)
Xu S, Jia Z (2007) Genome-wide analysis of epistatic effects for quantitative traits in barley. Genetics 175:1955–1963
Yu J, Pressoir G, Briggs WH, Bi IV, Yamasaki M, Doebley J, McMullen MD, Gaut BS, Nielsen DM, Holland JB, Kresovich S, Buckler ES (2006) A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat Genet 38:203–208
Zhao K, Aranzana MJ, Kim S, Lister C, Shindo C, Tang C, Toomajian C, Zheng H, Dean C, Marjoram P, Nordborg M (2007) An Arabidopsis example of association mapping in structured samples. PLoS Genet 3:e4
Acknowledgments
This research was conducted within the Biometric and Bioinformatic Tools for Genomics based Plant Breeding project supported by the German Federal Ministry of Education and Research (BMBF) within the framework of GABI–FUTURE initiative.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by X. Xia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reif, J.C., Maurer, H.P., Korzun, V. et al. Mapping QTLs with main and epistatic effects underlying grain yield and heading time in soft winter wheat. Theor Appl Genet 123, 283–292 (2011). https://doi.org/10.1007/s00122-011-1583-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-011-1583-y