Abstract
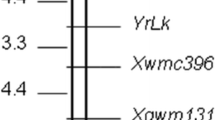
Stripe rust, caused by Puccinia striiformis f. sp. tritici, is one of the most widespread and destructive wheat diseases worldwide. Growing resistant cultivars is the preferred control of the disease. The spring wheat cultivar ‘Alpowa’ has both race-specific, all-stage resistance and non-race-specific, high-temperature adult-plant (HTAP) resistances to stripe rust. To identify genes for the stripe rust resistances, Alpowa was crossed with ‘Avocet Susceptible’ (AVS). Seedlings of the parents, and F1, F2 and F3 progeny were tested with races PST-1 and PST-21 of P. striiformis f. sp. tritici under controlled greenhouse conditions. Alpowa has a single partially dominant gene, designated as YrAlp, conferring all-stage resistance. Resistance gene analog polymorphism (RGAP) and simple sequence repeat (SSR) techniques were used to identify molecular markers linked to YrAlp. A linkage group of five RGAP markers and two SSR markers was constructed for YrAlp using 136 F3 lines. Amplification of a set of nulli-tetrasomic Chinese Spring lines with RGAP markers Xwgp47 and Xwgp48 and the two SSR markers indicated that YrAlp is located on the short arm of chromosome 1B. To map quantitative trait loci (QTLs) for the non-race-specific HTAP resistance, the parents and 136 F3 lines were tested at two sites near Pullman and one site near Mount Vernon, Washington, under naturally infected conditions. A major HTAP QTL was consistently detected across environments and was located on chromosome 7BL. Because of its chromosomal location and the non-race-specific nature of the HTAP resistance, this gene is different from previously described genes for adult-plant resistance, and is therefore designated Yr39. The gene contributed to 64.2% of the total variation of relative area under disease progress curve (AUDPC) data and 59.1% of the total variation of infection type data recorded at the heading-flowering stages. Two RGAP markers, Xwgp36 and Xwgp45 with the highest R 2 values were closely linked to Yr39, should be useful for incorporation of the non-race-specific resistance gene into new cultivars and for combining Yr39 with other genes for durable and high-level resistance.




Similar content being viewed by others
References
Allan RE, Purdy LH, Vogel OA (1966) Inheritance of seedling and adult reaction of wheat to stripe rust. Crop Sci 6:242–245
Börner A, Röder MS, Unger O, Meinel A (2000) The detection and molecular mapping of a major gene for non-specific adult-plant disease resistance against stripe rust (Puccinia striiformis) in wheat. Theor Appl Genet 100:1095–1099
Chen XM (2005) Epidemiology and control of stripe rust on wheat. Can J Plant Pathol 27:314–337
Chen XM, Line RF (1992a) Inheritance of stripe rust resistance in wheat cultivars used to differentiate races of Puccinia striiformis in North America. Phytopathology 82:633–637
Chen XM, Line RF (1992b) Identification of stripe rust resistance genes in wheat genotypes used to differentiate North American races of Puccinia striiformis. Phytopathology 82:1428–1434
Chen XM, Line RF (1995a) Gene action in wheat cultivars for durable high-temperature adult-plant resistance and interactions with race-specific, seedling resistance to stripe rust caused by Puccinia striiformis. Phytopathology 85:567–572
Chen XM, Line RF (1995b) Gene number and heritability of wheat cultivars with durable, high-temperature, adult-plant resistance and race-specific resistance to Puccinia striiformis. Phytopathology 85:573–578
Chen XM, Line RF, Hayes PM, Toojinda T, Vivar H, Kleinhofs A, Kudrna D (1999) Mapping barley genes for resistance to stripe rust, leaf rust, and scab using resistance gene analog polymorphism and restriction fragment length polymorphism. Phytopathology 89:S15
Chen XM, Line RF, Jones SS (1995) Chromosomal location of genes for stripe rust in spring wheat cultivars Compair, Fielder, Lee, and Lemhi and interactions of aneuploid wheats with races of Puccinia striiformis. Phytopathology 85:375–381
Chen XM, Line RF, Leung H (1998) Genome scanning for resistance-gene analogs in rice, barley, and wheat by high-resolution electrophoresis. Theor Appl Genet 97:345–355
Chen XM, Ling P, Wood DA, Moore MK, Pahalawatta V (2003) Epidemiology and control of wheat stripe rust in the United States, 2003. Ann Wheat Newsl 50:274–276
Chen XM, Moore M, Milus EA, Long DL, Line RF, Marshall D, Jackson L (2002) Wheat stripe rust epidemics and races of Puccinia striiformis f. sp. tritici in the United States in 2000. Plant Dis 86:39–46
Chicaiza O, Khan IA, Zhang X, Brevis CJ, Jackson L, Chen X, Dubcovsky J (2006) Registration of five wheat isogenic lines for leaf rust and stripe rust resistance genes. Crop Sci 46:485–487
Feuillet C, Schachermayr G, Keller B (1997) Molecular cloning of a new receptor-like kinase gene encoded at Lr10 disease resistance locus of wheat. Plant J 11:45–52
Geffroy V, Sevignac M, De Oliveira JCF, Fouilloux G, Skroch P, Thoquet P, Gepts P, Langin T, Dron M (2000) Inheritance of partial resistance against Colletotrichum lindemuthianum in Phaseolus vulgaris and co-localization of quantitative trait loci with genes involved in specific resistance. Mol Plant–Microbe Interact 13:287–296
Kanazin V, Marek LF, Shoemaker RC (1996) Resistance gene analogs are conserved and clustered in soybean. Proc Natl Acad Sci USA 93:11746–11750
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg I (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Leister D, Ballvora A, Salamini F, Gebhardt C (1996) A PCR-based approach for isolating pathogen resistance genes from potato with potential for wide application in plants. Nat Genet 14:421–429
Line RF (2002) Stripe rust of wheat and barley in North America: A retrospective historical review. Annu Rev Phytopathol 40:75–118
Line RF, Chen XM (1995) Successes in breeding for and managing durable resistance to wheat rusts. Plant Dis 79:1254–1255
Line RF, Qayoum A (1992) Virulence, aggressiveness, evolution, and distribution of races of Puccinia striiformis (the cause of stripe rust of wheat) in North America, 1968–87. U. S. Department of Agriculture Technical Bulletin No. 1788, p 44
McIntosh RA, Hart GE, Devos KM, Gale MD, Rogers WJ (1998) Catalogue of gene symbols for wheat. In: Slinkard AE (ed) Proceedings of the 9th international wheat genetic symposium, 2–7 August 1998, Saskatoon, Saskatchawan, Saskatchawan, Canada, vol 5. University Extension Press, University of Saskatchewan, Saskatoon, pp 1–235
McIntosh RA, Hart GE, Gale MD (1999) Catalogue of wheat symbols for wheat—1999 supplement (on line). In: Graingenes: a database for Triticeae and Avena. Available from http://www.wheat.pw.usda.gov/ggpages/pubs.html
McIntosh RA, Hart GE, Gale MD (2001) Catalogue of wheat symbols for wheat—2001 supplement (on line). In: Graingenes: a database for Triticeae and Avena. Available from http://www.wheat.pw.usda.gov/ggpages/pubs.html
McIntosh RA, Silk J, The TT (1996) Cytogenetic studies in wheat. XVII. Monosomic analysis and linkage relationships of gene Yr15 for resistance to stripe rust. Euphytica 89:395–399
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulk segregant analysis. A rapid method to detect markers in specific genomic regions by using segregating population. Proc Natl Acad Sci USA 88:9828–9832
Nelson JC (1997) Qgene: software for maker-based genomic analysis and breeding. Mol Breed 3:239–245
Pahalawatta V, Chen XM (2005a) Genetic analysis and molecular mapping of wheat genes conferring resistance to the wheat stripe rust and barley stripe rust pathogens. Phytopathology 95:427–432
Pahalawatta V, Chen XM (2005b) Inheritance and molecular mapping of barley genes conferring resistance to wheat stripe rust. Phytopathology 95:884–889
Peng JH, Fahima T, Roder MS, Li YC, Dahan A, Grama A, Ronin YI, Korol AB, Nevo E (1999) Microsatellite tagging of the stripe-rust resistance gene YrH52 derived from wild emmer wheat, Triticum dicoccoides, and suggestive negative crossover interference on chromosome 1B. Theor Appl Genet 98:862–872
Qayoum A, Line RF (1985) High-temperature, adult-plant resistance to stripe rust of wheat. Phytopathology 75:1121–1125
Rector BG, All JN, Parrott WA, Boerma HR (1998) Identification of molecular markers linked to quantitative trait loci for soybean resistance to corn earworm. Theor Appl Genet 96:786–790
Roder MS, Korzun V, Wendehake K, Plaschke J, Tixier MH, Leroy P, Ganal MW (1998) A microsatellite map of wheat. Genetics 149:207–333
Saghai-Maroof MA, Soliman K, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci USA 81:8014–8018
Santra DK, Santra M, Uauy C, Campbell KG, Chen XM, Dubcovsky J, Kidwell KK (2006) Identifying QTL for high-temperature adult-plant resistance to stripe rust in wheat (Triticum aestivum L.). Abstracts of Plant & Animal genome XIV, January 14–18, 2006, San Diego, CA, p 179
Sears ER (1966) Nullisomic–tetrasomic combinations in hexaploid wheat. In: Riley R, Lewis KR (eds) Chromosome manipulations and plant genetics. Oliver and Boyd, Edinburgh, pp 29–45
Shi ZX, Chen XM, Line RF, Leung H, Wellings CR (2001) Development of resistance gene analog polymorphism markers for the Yr9 gene resistance to wheat stripe rust. Genome 44:509–516
Stubbs RW (1985) Stripe rust. In: Roelfs AP, Bushnell WR (eds) The cereal rusts, vol II. Academic Press, New York, pp 61–101
Sun GL, Fahima T, Korol AB, Turpeinen T, Grama A, Ronin YI, Nevo E (1997) Identification of molecular markers linked to the Yr15 stripe rust resistance gene of wheat originated in wild emmer wheat Triticum dicoccoides. Theor Appl Genet 95:622–628
Toojinda T, Broers LH, Chen XM, Hayes PM, Kleinhofs A, Korte J, Kudrna D, Leung H, Line RF, Powell W, Ramsay L, Vivar H, Waugh R (2000) Mapping quantitative and qualitative disease resistance genes in a doubled haploid population of barley (Hordeum vulgare). Theor Appl Genet 101:580–589
Uauy C, Brevis JC, Chen XM, Khan IA, Jackson LF, Chicaiza O, Distelfeld A, Fahima T, Dubcovsky J (2005) High-temperature adult plant (HTAP) stripe rust resistance gene Yr36 from Triticum turgidum ssp. dicoccoides is closely linked to the grain protein content locus Gpc-B1. Theor Appl Genet 112:97–105
Wan AM, Zhao ZH, Chen XM, He ZH, Jin SL, Jia QZ, Yao G, Yang JX, Wang BT, Li GB, Bi YQ, Yuan ZY (2004) Wheat stripe rust epidemic and virulence of Puccinia striiformis f. sp. tritici in China in 2002. Plant Dis 88:896–904
Yan GP, Chen XM, Line RF, Wellings CR (2003) Resistance gene-analog polymorphism markers co-segregating with the Yr5 gene for resistance to wheat stripe rust. Theor Appl Genet 106:636–643
Yu YG, Buss GR, Maroof MA (1996) Isolation of a superfamily of candidate disease resistance genes in soybean based on a conserved nucleotide-binding site. Proc Natl Acad Sci USA 93:11751–11756
Acknowledgments
This research was supported by the US Department of Agriculture, Agricultural Research Service, Vogel Foundation and Washington Wheat Commission. PPNS No. 0421, Department of Plant Pathology, College of Agricultural, Human, and Natural Resource Sciences Research Center, Project Nos. 13Z-3061-3824 and 13C-30613923, Washington State University, Pullman, WA 99164-6430, USA. We are grateful to D. Wood, L. Penman, Y.M. Liu, V. Pahalawatta, Dr. P. Ling, Dr. D. Santra and Dr. L. Reddy for their technical assistance. We also would like to thank Drs. R.E. Allan, R.F. Line and R.A. McIntosh for their critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Graner.
Rights and permissions
About this article
Cite this article
Lin, F., Chen, X.M. Genetics and molecular mapping of genes for race-specific all-stage resistance and non-race-specific high-temperature adult-plant resistance to stripe rust in spring wheat cultivar Alpowa. Theor Appl Genet 114, 1277–1287 (2007). https://doi.org/10.1007/s00122-007-0518-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-007-0518-0