Abstract
It has been recently reported that cigarette smoke exposure during allergen sensitization facilitates the development of allergic asthma; however, the underlying mechanisms remain elusive. We evaluated the role of interleukin (IL-23) in a cigarette smoke extract (CSE)-induced Dermatophagoides pteronyssinus (Dp)-allergic asthma mouse model. BALB/c mice were exposed to CSE during allergen sensitization period. Anti-IL-23p19 or IL-23R antibody was administered during the sensitization period. And we evaluated several immunological responses. The expression of IL-23 and IL-23 receptor (IL-23R) was examined in lung tissue. IL-23 and IL-23R expression was increased in the airway epithelium of Dp/CSE co-administered mice. CSE administration during the sensitization promoted Dp-allergic sensitization and the development of asthma phenotypes. Additionally, the proportion of innate lymphoid type 2 cells (ILC2) was also increased by CSE and Dp co-instillation. Anti-IL-23 or IL-23R antibody treatment during allergen sensitization significantly diminished phenotypes of allergic asthma and the ILC2 population. The levels of IL-33 and thymic stromal lymphopoietin (TSLP) were also significantly reduced by anti-IL-23 or IL-23R antibody treatment. IL-23 may thus play a significant role in cigarette smoke-induced allergic sensitization and asthma development. Clinically, the increase in allergen sensitization due to cigarette exposure causes onset of asthma, and IL-23 may be important in this mechanism.
Key messages
-
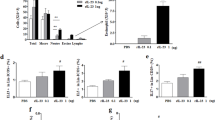
IL-23 and IL-23R expression was increased in the lung epithelium of Dp and CSE co-exposed mice during sensitization period.
-
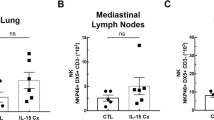
The population of ILC2s was increased in Dp and CSE co-exposed mice during sensitization period.
-
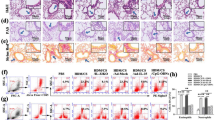
Anti-IL23 or IL-23R antibody treatment with co-administration of CSE and HDM during sensitization period significantly suppresses ILC2.
-
In vitro, IL-23 blockade in Dp and CSE-stimulated epithelial cells suppressed IL-13 expression in ILC2.








Similar content being viewed by others
Change history
22 May 2019
The original publication of this paper contains a mistake.
References
D’Amato G, Liccardi G, D’Amato M, Holgate S (2005) Environmental risk factors and allergic bronchial asthma. Clin Exp Allergy 35:1113–1124
Chan-Yeung M, Dimich-Ward H (2003) Respiratory health effects of exposure to environmental tobacco smoke. Respirology 8:131–139
Coultas DB (1998) Health effects of passive smoking. 8. Passive smoking and risk of adult asthma and COPD: an update. Thorax 53:381–387
Thomson NC, Chaudhuri R, Livingston E (2004) Asthma and cigarette smoking. Eur Respir J 24:822–833
Polosa R, Russo C, Caponnetto P, Bertino G, Sarva M, Antic T, Mancuso S, Al-Delaimy WK (2011) Greater severity of new onset asthma in allergic subjects who smoke: a 10-year longitudinal study. Respir Res 12:16
Comhair SA, Gaston BM, Ricci KS, Hammel J, Dweik RA, Teague WG, Meyers D, Ampleford EJ, Bleecker ER, Busse WW et al (2011) Detrimental effects of environmental tobacco smoke in relation to asthma severity. PLoS One 6:e18574
Jarvis D, Chinn S, Luczynska C, Burney P (1999) The association of smoking with sensitization to common environmental allergens: results from the European Community Respiratory Health Survey. J Allergy Clin Immunol 104:934–940
Lanckacker EA, Tournoy KG, Hammad H, Holtappels G, Lambrecht BN, Joos GF, Maes T (2013) Short cigarette smoke exposure facilitates sensitisation and asthma development in mice. Eur Respir J 41:1189–1199
Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE et al (2013) Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol 13:145–149
Doherty TA (2015) At the bench: understanding group 2 innate lymphoid cells in disease. J Leukoc Biol 97:455–467
Moerloose KB, Robays LJ, Maes T, Brusselle GG, Tournoy KG, Joos GF (2006) Cigarette smoke exposure facilitates allergic sensitization in mice. Respir Res 7:49
Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K et al (2000) Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715–725
Lankford CS, Frucht DM (2003) A unique role for IL-23 in promoting cellular immunity. J Leukoc Biol 73:49–56
Kopp T, Lenz P, Bello-Fernandez C, Kastelein RA, Kupper TS, Stingl G (2003) IL-23 production by cosecretion of endogenous p19 and transgenic p40 in keratin 14/p40 transgenic mice: evidence for enhanced cutaneous immunity. J Immunol 170:5438–5444
Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, Lucian L, Geissler R, Brodie S, Kimball AB et al (2006) IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med 203:2577–2587
Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez-Farinas M, Fuentes-Duculan J, Novitskaya I, Khatcherian A, Bluth MJ, Lowes MA et al (2007) Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med 204:3183–3194
Piskin G, Tursen U, Sylva-Steenland RM, Bos JD, Teunissen MB (2004) Clinical improvement in chronic plaque-type psoriasis lesions after narrow-band UVB therapy is accompanied by a decrease in the expression of IFN-gamma inducers -- IL-12, IL-18 and IL-23. Exp Dermatol 13:764–772
Peng J, Yang XO, Chang SH, Yang J, Dong C (2010) IL-23 signaling enhances Th2 polarization and regulates allergic airway inflammation. Cell Res 20:62–71
Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y et al (2008) IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med 178:1023–1032
Lee HS, Park HW, Song WJ, Jeon EY, Bang B, Shim EJ, Moon HG, Kim YK, Kang HR, Min KU et al (2016) TNF-alpha enhance Th2 and Th17 immune responses regulating by IL23 during sensitization in asthma model. Cytokine 79:23–30
Lee HS, Park DE, Lee JW, Chang Y, Kim HY, Song WJ, Kang HR, Park HW, Chang YS, Cho SH (2017) IL-23 secreted by bronchial epithelial cells contributes to allergic sensitization in asthma model: role of IL-23 secreted by bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 312:L13–L21
Cornelissen F, Asmawidjaja PS, Mus AM, Corneth O, Kikly K, Lubberts E (2013) IL-23 dependent and independent stages of experimental arthritis: no clinical effect of therapeutic IL-23p19 inhibition in collagen-induced arthritis. PLoS One 8:e57553
Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Berard M, Kleinschek M, Cua D, Di Santo JP, Eberl G (2011) RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol 12:320–326
Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H (2012) IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol 188:1503–1513
Halim TY, Krauss RH, Sun AC, Takei F (2012) Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 36:451–463
Martinez-Gonzalez I, Steer CA, Takei F (2015) Lung ILC2s link innate and adaptive responses in allergic inflammation. Trends Immunol 36:189–195
Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F et al (2002) A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol 168:5699–5708
McKenzie AN (2014) Type-2 innate lymphoid cells in asthma and allergy. Ann Am Thorac Soc 11(Suppl 5):S263–S270
Jia Y, Fang X, Zhu X, Bai C, Zhu L, Jin M, Wang X, Hu M, Tang R, Chen Z (2016) IL-13(+) type 2 innate lymphoid cells correlate with asthma control status and treatment response. Am J Respir Cell Mol Biol 55:675–683
Abraham C, Cho JH (2009) IL-23 and autoimmunity: new insights into the pathogenesis of inflammatory bowel disease. Annu Rev Med 60:97–110
Di Cesare A, Di Meglio P, Nestle FO (2009) The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Investig Dermatol 129:1339–1350
Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, Matsunami N, Ardlie KG, Civello D, Catanese JJ et al (2007) A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet 80:273–290
Maldonado-Lopez R, Moser M (2001) Dendritic cell subsets and the regulation of Th1/Th2 responses. Semin Immunol 13:275–282
Cates EC, Fattouh R, Wattie J, Inman MD, Goncharova S, Coyle AJ, Gutierrez-Ramos JC, Jordana M (2004) Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol 173:6384–6392
Halim TYF, McKenzie ANJ (2013) New kids on the block: group 2 innate lymphoid cells and type 2 inflammation in the lung. Chest 144:1681–1686
Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T et al (2003) Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421:744–748
McKenzie ANJ, Spits H, Eberl G (2014) Innate lymphoid cells in inflammation and immunity. Immunity 41:366–374
Huang GO, Belfort MA, Whitehead WE, Olutoye OO, Castillo J, Castillo H, Ostermaier KK, Koh CJ, Tu DD (2017) Early postnatal bladder function in fetoscopic myelomeningocele repair patients. J Pediatr Rehabil Med 10:327–333
Kumar S, Lanckacker E, Dentener M, Bracke K, Provoost S, De Grove K, Brusselle G, Wouters E, Maes T, Joos G (2016) Aggravation of allergic airway inflammation by cigarette smoke in mice is CD44-dependent. PLoS One 11:e0151113
Elliott MK, Sisson JH, West WW, Wyatt TA (2006) Differential in vivo effects of whole cigarette smoke exposure versus cigarette smoke extract on mouse ciliated tracheal epithelium. Exp Lung Res 32:99–118
Nakamura Y, Miyata M, Ohba T, Ando T, Hatsushika K, Suenaga F, Shimokawa N, Ohnuma Y, Katoh R, Ogawa H et al (2008) Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J Allergy Clin Immunol 122:1208–1214
Trimble NJ, Botelho FM, Bauer CM, Fattouh R, Stampfli MR (2009) Adjuvant and anti-inflammatory properties of cigarette smoke in murine allergic airway inflammation. Am J Respir Cell Mol Biol 40:38–46
Botelho FM, Llop-Guevara A, Trimble NJ, Nikota JK, Bauer CM, Lambert KN, Kianpour S, Jordana M, Stampfli MR (2011) Cigarette smoke differentially affects eosinophilia and remodeling in a model of house dust mite asthma. Am J Respir Cell Mol Biol 45:753–760
Alyasin S, Amin R, Fazel A, Karimi MH, Nabavizadeh SH, Esmaeilzadeh H, Babaei M (2017) IL-23 gene and protein expression in childhood asthma. Iranian J Immunol 14:73–80
Abdollahi E, Tavasolian F, Momtazi-Borojeni AA, Samadi M, Rafatpanah H (2016) Protective role of R381Q (rs11209026) polymorphism in IL-23R gene in immune-mediated diseases: a comprehensive review. J Immunotoxicol 13:286–300
Kim HY, Shin YH, Han MY (2014) Determinants of sensitization to allergen in infants and young children. Korean J Pediatr 57:205–210
Gold MJ, Antignano F, Halim TY, Hirota JA, Blanchet MR, Zaph C, Takei F, McNagny KM (2014) Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. J Allergy Clin Immunol 133:1142–1148
Licona-Limon P, Kim LK, Palm NW, Flavell RA (2013) TH2, allergy and group 2 innate lymphoid cells. Nat Immunol 14:536–542
Acknowledgements
We thank Hee-seung Lee for her comments on this manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2016R1C1B1010632).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were performed with the approval of the Institutional Animal Care and Use Committee of the Institute (IACUC) of Seoul National University. The approval number is SNU-160615-5-4.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The original publication of this paper contains a mistake. Correct images for figures 1,2, 3, 4 and 5 are shown in this paper.
Electronic supplementary material
ESM 1
(DOCX 356 kb)
Rights and permissions
About this article
Cite this article
Lee, H.S., Park, DE., Lee, JW. et al. Critical role of interleukin-23 in development of asthma promoted by cigarette smoke. J Mol Med 97, 937–949 (2019). https://doi.org/10.1007/s00109-019-01768-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-019-01768-y