Abstract
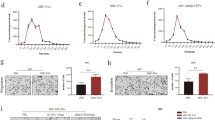
The capacity of cancer cells to undergo epithelial-to-mesenchymal transition (EMT) is now considered a hallmark of tumor progression, and it is known that interactions between cancer cells and mesenchymal stem cells (MSCs) of tumor microenvironment may promote this program. Herein, we demonstrate that MSC-conditioned medium (MSC-CM) is a potent inducer of EMT in melanoma cells. The EMT profile acquired by MSC-CM-exposed melanoma cells is characterized by an enhanced level of mesenchymal markers, including TGFβ/TGFβ-receptors system upregulation, by increased invasiveness and uPAR expression, and in vivo tumor growth. Silencing TGFβ in MSC is found to abrogate ability of MSC to promote EMT characteristics in melanoma cells, together with uPAR expression, and this finding is strengthened using an antagonist peptide of TGFβRIII, the so-called P17. Finally, we demonstrate that the uPAR antisense oligonucleotide (uPAR aODN) may inhibit EMT of melanoma cells either stimulated by exogenous TGFβ or MSC-CM. Thus, uPAR upregulation in melanoma cells exposed to MSC-medium drives TGFβ-mediated EMT. On the whole, TGFβ/uPAR dangerous liaison in cancer cell/MSC interactions may disclose a new strategy to abrogate melanoma progression.
Key message
-
Mesenchymal stem cell (MSC)-conditioned medium induces EMT-like profile in melanoma.
-
MSC-derived TGFβ promotes uPAR and TGFβ/TGFβ-receptor upregulation in melanoma.
-
TGFβ gene silencing in MSCs downregulates uPAR expression and EMT in melanoma.
-
uPAR downregulation prevents MSC-induced EMT-like profile in melanoma cells.
-
Inhibition of the dangerous TGFβ/uPAR relationship might abrogate melanoma progression.






Similar content being viewed by others
Abbreviations
- MSC:
-
Mesenchymal stem cells
- EMT:
-
Epithelial-to-mesenchymal transition
- TGFβ:
-
Transforming growth factor beta
- uPAR:
-
Urokinase-type plasminogen activator receptor
- uPAR aODN:
-
uPAR antisense oligonucleotide
- MSC-CM:
-
Medium conditioned by mesenchymal stem cells
References
Chamberlain G, Fox J, Ashton B, Middleton J (2007) Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25:2739–2749
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449:557–563
Martin FT, Dwyer RM, Kelly J, Khan S, Murphy JM, Curran C, Miller N, Hennessy E, Dockery P, Barry FP et al (2010) Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res Treat 124:317–326
Shinagawa K, Kitadai Y, Tanaka M, Sumida T, Kodama M, Higashi Y, Tanaka S, Yasui W, Chayama K (2010) Mesenchymal stem cells enhance growth and metastasis of colon cancer. Int J Cancer 127:2323–2333
Mele V, Muraro MG, Calabrese D, Pfaff D, Amatruda N, Amicarella F, Kvinlaug B, Bocelli-Tyndall C, Martin I, Resink TJ et al (2014) Mesenchymal stromal cells induce epithelial-to-mesenchymal transition in human colorectal cancer cells through the expression of surface-bound TGF-β. Int J Cancer 134:2583–2594
Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G et al (2006) Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med 203:1235–1247
Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, Cao W, Han C, Chen Y (2006) Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol 80:267–274
Chanda D, Isayeva T, Kumar S, Hensel JA, Sawant A, Ramaswamy G, Siegal GP, Beatty MS, Ponnazhagan S (2009) Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in prostate cancer bone metastasis. Clin Cancer Res 15:7175–7185
Ye H, Cheng J, Tang Y, Liu Z, Xu C, Liu Y, Sun Y (2012) Human bone marrow-derived mesenchymal stem cells produced TGFbeta contributes to progression and metastasis of prostate cancer. Cancer Invest 30:513–518
Massagué J (2008) TGFbeta in cancer. Cell 134:215–230
Tian M, Neil JR, Schiemann WP (2011) Transforming growth factor-β and the hallmarks of cancer. Cell Signal 23:951–962
Katz LH, Li Y, Chen JS, Muñoz NM, Majumdar A, Chen J, Mishra L (2013) Targeting TGF-β signaling in cancer. Expert Opin Ther Targets 17:743–760
Serratì S, Margheri F, Pucci M, Cantelmo AR, Cammarota R, Dotor J, Borràs-Cuesta F, Fibbi G, Albini A, Del Rosso M (2009) TGFbeta1 antagonistic peptides inhibit TGFbeta1-dependent angiogenesis. Biochem Pharmacol 77:813–825
Santibanez JF (2013) Transforming growth factor-Beta and urokinase-type plasminogen activator: dangerous partners in tumorigenesis-implications in skin cancer. Dermatol 597927
Schmitt M, Mengele K, Napieralski R, Magdolen V, Reuning U, Gkazepis A, Sweep F, Brünner N, Foekens J, Harbeck N (2010) Clinical utility of level-of-evidence-1 disease forecast cancer biomarkers uPA and its inhibitor PAI-1. Expert Rev Mol Diagn 10:1051–1067
Jo M, Lester RD, Montel V, Eastman B, Takimoto S, Gonias SL (2009) Reversibility of epithelial-mesenchymal transition (EMT) induced in breast cancer cells by activation of urokinase receptor-dependent cell signaling. J Biol Chem 284:22825–22833
Lester RD, Jo M, Montel V, Takimoto S, Gonias SL (2007) uPAR induces epithelial-mesenchymal transition in hypoxic breast cancer cells. J Cell Biol 178:425–436
Alonso SR, Tracey L, Ortiz P, Pérez-Gómez B, Palacios J, Pollán M, Linares J, Serrano S, Sáez-Castillo AI, Sánchez L et al (2007) A high-throughput study in melanoma identifies epithelial-mesenchymal transition as a major determinant of metastasis. Cancer Res 67:3450–3460
Laurenzana A, Biagioni A, D’Alessio S, Bianchini F, Chillà A, Margheri F, Luciani C, Mazzanti B, Pimpinelli N, Torre E et al (2014) Melanoma cell therapy: endothelial progenitor cells as shuttle of the MMP12 uPAR-degrading enzyme. Oncotarget 5:3711–3727
Peppicelli S, Bianchini F, Contena C, Tombaccini D, Calorini L (2013) Acidic pH via NF-κB favours VEGF-C expression in human melanoma cells. Clin Exp Metastasis 30:957–967
Dotor J, López-Vázquez AB, Lasarte JJ, Sarobe P, García-Granero M, Riezu-Boj JI, Martínez A, Feijoó E, López-Sagaseta J, Hermida J et al (2007) Identification of peptide inhibitors of transforming growth factor beta 1 using a phage-displayed peptide library. Cytokine 39:106–115
Margheri F, Schiavone N, Papucci L, Magnelli L, Serratì S, Chillà A, Laurenzana A, Bianchini F, Calorini L, Torre E et al (2012) GDF5 regulates TGFß-dependent angiogenesis in breast carcinoma MCF-7 cells: in vitro and in vivo control by anti-TGFß peptides. PLoS One 7:e50342
Margheri F, Luciani C, Taddei ML, Giannoni E, Laurenzana A, Biagioni A, Chillà A, Chiarugi P, Fibbi G, Del Rosso M (2014) The receptor for urokinase-plasminogen activator (uPAR) controls plasticity of cancer cell movement in mesenchymal and amoeboid migration style. Oncotarget 5:1538–1553
D’Alessio S, Margheri F, Pucci M, Del Rosso A, Monia BP, Bologna M, Leonetti C, Scarsella M, Zupi G, Fibbi G et al (2004) Antisense oligodeoxynucleotides for urokinase-plasminogen activator receptor have anti-invasive and anti-proliferative effects in vitro and inhibit spontaneous metastases of human melanoma in mice. Int J Cancer 110(1):125–133
Margheri F, Chillà A, Laurenzana A, Serratì S, Mazzanti B, Saccardi R, Santosuosso M, Danza G, Sturli N, Rosati F et al (2011) Endothelial progenitor cell-dependent angiogenesis requires localization of the full-length form of uPAR in caveolae. Blood 118:3743–3755
Laurenzana A, Balliu M, Cellai C, Romanelli MN, Paoletti F (2013) Effectiveness of the histone deacetylase inhibitor (S)-2 against LNCaP and PC3 human prostate cancer cells. PLoS One 8:e5826
Quattrone A, Fibbi G, Anichini E, Pucci M, Zamperini A, Capaccioli S, Del Rosso M (1995) Reversion of the invasive phenotype of transformed human fibroblasts by anti-messenger oligonucleotide inhibition of urokinase receptor gene expression. Cancer Res 55:90–95
Andreasen PA, Egelund R, Petersen HH (2000) The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci 57:25–40
Danø K, Behrendt N, Høyer-Hansen G, Johnsen M, Lund LR, Ploug M, Rømer J (2005) Plasminogen activation and cancer. Thromb Haemost 93:676–681
Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH (2008) Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev 34:122–136
Freudlsperger C, Bian Y, Contag Wise S, Burnett J, Coupar J, Yang X, Chen Z, Van Waes C (2013) TGF-β and NF-κB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene 32:1549–1559
Roberts AB, Wakefield LM (2003) The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A 100:8621–8623
Moustakas A, Heldin P (2014) TGFβ and matrix-regulated epithelial to mesenchymal transition. Biochim Biophys Acta S0304–4165(14):00050–00056
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15:178–196
Schlegel NC, von Planta A, Widmer DS, Dummer R, Christofori G (2015) PI3K signalling is required for a TGFβ-induced epithelial-mesenchymal-like transition (EMT-like) in human melanoma cells. Exp Dermatol 24:22–28
Hoek KS, Schlegel NC, Brafford P, Sucker A, Ugurel S, Kumar R, Weber BL, Nathanson KL, Phillips DJ, Herlyn M et al (2006) Metastatic potential of melanomas defined by specific gene expression profiles with no BRAF signature. Pigment Cell Res 19:290–302
Goldstein RH, Reagan MR, Anderson K, Kaplan DL, Rosenblatt M (2010) Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res 70:10044–10050
Mazar AP, Ahn RW, O’Halloran TV (2011) Development of novel therapeutics targeting the urokinase plasminogen activator receptor (uPAR) and their translation toward the clinic. Curr Pharm Des 17:1970–1978
Del Rosso M, Fibbi G, Pucci M, D’Alessio S, Del Rosso A, Magnelli L, Chiarugi V (2002) Multiple pathways of cell invasion are regulated by multiple families of serine proteases. Clin Exp Metastasis 19:193–207
Fibbi G, Caldini R, Chevanne M, Pucci M, Schiavone N, Morbidelli L, Parenti A, Granger HJ, Del Rosso M, Ziche M (1988) Urokinase-dependent angiogenesis in vitro and diacylglycerol production are blocked by antisense oligonucleotides against the urokinase receptor. Lab Invest 78:1109–1119
Fibbi G, Ziche M, Morbidelli L, Magnelli L, Del Rosso M (1998) Interaction of urokinase with specific receptors stimulates mobilization of bovine adrenal capillary endothelial cells. Exp Cell Res 179:385–395
Rao JS, Gondi C, Chetty C, Chittivelu S, Joseph PA, Lakka SS (2005) Inhibition of invasion, angiogenesis, tumor growth, and metastasis by adenovirus-mediated transfer of antisense uPAR and MMP-9 in non-small cell lung cancer cells. Mol Cancer Ther 4:1399–1408
Acknowledgments
Financial support: Istituto Toscano Tumori (MDR), Ente Cassa di Risparmio di Firenze (GF, LC), and Associazione Italiana Ricerca sul Cancro (AIRC), grant IG 2013 N. 14266 (MDR). F.M. was supported by a post-doctoral fellowship of the European Union in the project UNIFI-4 MELOTAC (Italian acronym: UNIFI_FSE2012).
Conflict of interest
The authors state no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Anna Laurenzana and Alessio Biagioni contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 639 kb)
Rights and permissions
About this article
Cite this article
Laurenzana, A., Biagioni, A., Bianchini, F. et al. Inhibition of uPAR-TGFβ crosstalk blocks MSC-dependent EMT in melanoma cells. J Mol Med 93, 783–794 (2015). https://doi.org/10.1007/s00109-015-1266-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-015-1266-2