Abstract
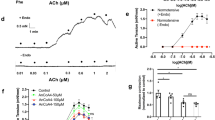
Endothelial cell-dependent vascular relaxation plays an important role in the regulation of blood pressure. Here, we show that stimulation of vascular endothelial cells with platelet-derived growth factor (PDGF) results in vascular relaxation through Akt1-dependent activation of endothelial nitric oxide synthase (eNOS) and nitric oxide (NO) production. Stimulation of both human umbilical artery endothelial cells and abdominal aortic vessels with PDGF induced NO production. PDGF-dependent production of NO was completely abolished by inhibition of phosphatidylinositol 3-kinase with wortmannin (100 nM). Stimulation of aortic vessels with PDGF resulted in the activation of Akt phosphorylation and eNOS phosphorylation: however, eNOS phosphorylation and production of NO were abolished in aortic vessels of mice lacking Akt1. PDGF strongly induced vascular relaxation in the presence of endothelium, and inhibition of NO production by N-nitro-l-arginine-methyl ester completely blocked PDGF-dependent vascular relaxation. In addition, PDGF-dependent relaxation was completely abolished by inhibition of PI3K with wortmannin (100 nM). Furthermore, vessels from Akt1 heterozygotes showed normal relaxation after PDGF stimulation, whereas vessels from Akt1 knockout littermates did not respond to PDGF stimulation. Finally, administration of PDGF (5 ng/ml) significantly lowered blood pressure in Akt1 heterozygotes, whereas a blood pressure-lowering effect was not observed in Akt1 knockout littermates. These results suggest that Akt1 regulates blood pressure through regulation of vascular relaxation by eNOS phosphorylation and subsequent production of NO.





Similar content being viewed by others
References
Cooke JP, Losordo DW (2002) Nitric oxide and angiogenesis. Circulation 105:2133–2135
Mount PF, Kemp BE, Power DA (2007) Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 42:271–279
Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR and Kemp BE (1999) AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443:285–289
Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R (2001) Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88:E68–E75
Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM (1999) Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601–605
Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A and Sessa WC (1999) Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399:597–601
Michell BJ, Griffiths JE, Mitchelhill KI, Rodriguez-Crespo I, Tiganis T, Bozinovski S, de Montellano PR, Kemp BE and Pearson RB (1999) The Akt kinase signals directly to endothelial nitric oxide synthase. Curr Biol 9:845–848
Brazil DP, Yang ZZ, Hemmings BA (2004) Advances in protein kinase B signalling: AKT ion on multiple fronts. Trends Biochem Sci 29:233–242
Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN (1995) The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81:727–736
Takata M, Ogawa W, Kitamura T, Hino Y, Kuroda S, Kotani K, Klip A, Gingras AC, Sonenberg N and Kasuga M (1999) Requirement for Akt (protein kinase B) in insulin-induced activation of glycogen synthase and phosphorylation of 4E-BP1 (PHAS-1). J Biol Chem 274:20611–20618
Zheng WH, Quirion R (2006) Insulin-like growth factor-1 (IGF-1) induces the activation/phosphorylation of Akt kinase and cAMP response element-binding protein (CREB) by activating different signaling pathways in PC12 cells. BMC Neurosci 7:51
Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB 3rd, Kaestner KH, Bartolomei MS, Shulman GI and Birnbaum MJ (2001) Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB β). Science 292:1728–1731
Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T et al. (2005) Role for Akt3/protein kinase Bγ in attainment of normal brain size. Mol Cell Biol 25:1869–1878
Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, Michaelis T, Frahm J and Hemmings BA (2005) Essential role of protein kinase B gamma (PKBγ/Akt3) in postnatal brain development but not in glucose homeostasis. Development 132:2943–2954
Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ (2001) Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem 276:38349–38352
Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ et al. (2005) Akt1/protein kinase Bα is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest 115:2119–2127
Schleicher M, Yu J, Murata T, Derakhshan B, Atochin D, Qian L, Kashiwagi S, Di Lorenzo A, Harrison KD, Huang PL et al. (2009) The Akt1-eNOS axis illustrates the specificity of kinase-substrate relationships in vivo. Sci Signal 2(82):ra41
Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ and Sessa WC (2007) Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab 6:446–457
Claesson-Welsh L (1994) Platelet-derived growth factor receptor signals. J Biol Chem 269:32023–32026
Majesky MW, Reidy MA, Bowen-Pope DF, Hart CE, Wilcox JN, Schwartz SM (1990) PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol 111:2149–2158
Schwartz SM, Heimark RL, Majesky MW (1990) Developmental mechanisms underlying pathology of arteries. Physiol Rev 70:1177–1209
Koyama N, Morisaki N, Saito Y, Yoshida S (1992) Regulatory effects of platelet-derived growth factor-AA homodimer on migration of vascular smooth muscle cells. J Biol Chem 267:22806–22812
Berk BC, Alexander RW, Brock TA, Gimbrone MA Jr, Webb RC (1986) Vasoconstriction: a new activity for platelet-derived growth factor. Science 232:87–90
Beitz JG, Kim IS, Calabresi P, Frackelton AR Jr (1991) Human microvascular endothelial cells express receptors for platelet-derived growth factor. Proc Natl Acad Sci U S A 88:2021–2025
Cunningham LD, Brecher P, Cohen RA (1992) Platelet-derived growth factor receptors on macrovascular endothelial cells mediate relaxation via nitric oxide in rat aorta. J Clin Invest 89:878–882
Haudenschild CC, Zahniser D, Folkman J, Klagsbrun M (1976) Human vascular endothelial cells in culture. Lack of response to serum growth factors. Exp Cell Res 98:175–183
Bae SS, Cho H, Mu J, Birnbaum MJ (2003) Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem 278:49530–49536
Thors B, Halldorsson H, Thorgeirsson G (2004) Thrombin and histamine stimulate endothelial nitric-oxide synthase phosphorylation at Ser1177 via an AMPK mediated pathway independent of PI3K-Akt. FEBS Lett 573:175–180
Ohashi Y, Kawashima S, Hirata K, Yamashita T, Ishida T, Inoue N, Sakoda T, Kurihara H, Yazaki Y and Yokoyama M (1998) Hypotension and reduced nitric oxide-elicited vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. J Clin Invest 102:2061–2071
Luo Z, Fujio Y, Kureishi Y, Rudic RD, Daumerie G, Fulton D, Sessa WC, Walsh K (2000) Acute modulation of endothelial Akt/PKB activity alters nitric oxide-dependent vasomotor activity in vivo. J Clin Invest 106:493–499
Selimovic N, Bergh CH, Andersson B, Sakiniene E, Carlsten H, Rundqvist B (2009) Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J 34:662–668
Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA et al. (2009) Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res 104:1085–1094
Acknowledgments
This work was supported partly by the MRC program of the NRF (2011-0006188) and a National Research Foundation of Korea grant funded by the Korea government (MEST) (2010-0015808).
Disclosures/conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jung Min Ha and Young Whan Kim contributed equally to this work.
An erratum to this article can be found online at http://dx.doi.org/10.1007/s00109-013-1023-3.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
PDF 3349 kb
Rights and permissions
About this article
Cite this article
Ha, J.M., Kim, Y.W., Lee, D.H. et al. Regulation of arterial blood pressure by Akt1-dependent vascular relaxation. J Mol Med 89, 1253–1260 (2011). https://doi.org/10.1007/s00109-011-0798-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-011-0798-3