Abstract
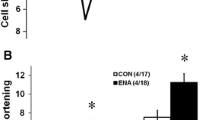
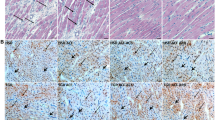
Renin-angiotensin system (RAS) activation is associated with arrhythmias. We investigated the effects of RAS inhibition in cardiac-specific angiotensin-converting enzyme (ACE) overexpression (ACE 8/8) mice, which exhibit proclivity to ventricular tachycardia (VT) and sudden death because of reduced connexin43 (Cx43). ACE 8/8 mice were treated with an ACE inhibitor (captopril) or an angiotensin receptor type-1 blocker (losartan). Subsequently, electrophysiological studies were performed, and the hearts were extracted for Cx43 quantification using immunoblotting, immunohistochemistry, fluorescent dye spread method, and sodium current quantification using whole cell patch clamping. VT was induced in 12.5% of captopril-treated ACE 8/8 and in 28.6% of losartan-treated mice compared to 87.5% of untreated mice (P < 0.01). Losartan and captopril treatment increased total Cx43 2.4-fold (P = 0.01) and the Cx43 phosphorylation ratio 2.3-fold (P = 0.005). Treatment was associated with a recovery of gap junctional conductance. Survival in treated mice improved to 0.78 at 10 weeks (95% confidence interval 0.64 to 0.92), compared to the expected survival of less than 0.50. In a model of RAS activation, arrhythmic risk was correlated with reduced Cx43 amount and phosphorylation. RAS inhibition resulted in increased total and phosphorylated Cx43, decreased VT inducibility, and improved survival.






Similar content being viewed by others
References
Swedberg K, Kjekshus J (1988) Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol 62:60A–66A
Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G (2000) Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients the heart outcomes prevention evaluation study investigators. N Engl J Med 342:145–153
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure randomized aldactone evaluation study investigators. N Engl J Med 341:709–717
Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M (2003) Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348:1309–1321
Crabos M, Roth M, Hahn AW, Erne P (1994) Characterization of angiotensin II receptors in cultured adult rat cardiac fibroblasts. Coupling to signaling systems and gene expression. J Clin Invest 93:2372–2378
Kaibara M, Mitarai S, Yano K, Kameyama M (1994) Involvement of Na+-H+ antiporter in regulation of L-type Ca2+ channel current by angiotensin II in rabbit ventricular myocytes. Circ Res 75:1121–1125
Caballero R, Gomez R, Moreno I, Nunez L, Gonzalez T, Arias C, Guizy M, Valenzuela C, Tamargo J, Delpon E (2004) Interaction of angiotensin II with the angiotensin type 2 receptor inhibits the cardiac transient outward potassium current. Cardiovasc Res 62:86–95
Ju H, Scammel-La Fleur T, Dixon IM (1996) Altered mRNA abundance of calcium transport genes in cardiac myocytes induced by angiotensin II. J Mol Cell Cardiol 28:1119–1128
Tokuhisa T, Yano M, Obayashi M, Noma T, Mochizuki M, Oda T, Okuda S, Doi M, Liu J, Ikeda Y et al (2006) AT1 receptor antagonist restores cardiac ryanodine receptor function, rendering isoproterenol-induced failing heart less susceptible to Ca2+ -leak induced by oxidative stress. Circ J 70:777–786
Flesch M, Schiffer F, Zolk O, Pinto Y, Stasch JP, Knorr A, Ettelbruck S, Bohm M (1997) Angiotensin receptor antagonism and angiotensin converting enzyme inhibition improve diastolic dysfunction and Ca(2+)-ATPase expression in the sarcoplasmic reticulum in hypertensive cardiomyopathy. J Hypertens 15:1001–1009
Xiao HD, Fuchs S, Campbell DJ, Lewis W, Dudley SC Jr, Kasi VS, Hoit BD, Keshelava G, Zhao H, Capecchi MR et al (2004) Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol 165:1019–1032
Kasi VS, Xiao HD, Shang LL, Iravanian S, Langberg J, Witham EA, Jiao Z, Gallego CJ, Bernstein KE, Dudley SC (2007) Cardiac restricted angiotensin converting enzyme overexpression causes conduction defects and connexin dysregulation. Am J Physiol Heart Circ Physiol 293:H182–H192
van Veen AA, van Rijen HV, Opthof T (2001) Cardiac gap junction channels: modulation of expression and channel properties. Cardiovasc Res 51:217–229
Shaw RM, Rudy Y (1997) Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res 81:727–741
Kaprielian RR, Gunning M, Dupont E, Sheppard MN, Rothery SM, Underwood R, Pennell DJ, Fox K, Pepper J, Poole-Wilson PA et al (1998) Downregulation of immunodetectable connexin43 and decreased gap junction size in the pathogenesis of chronic hibernation in the human left ventricle. Circulation 97:651–660
Teunissen BE, Jongsma HJ, Bierhuizen MF (2004) Regulation of myocardial connexins during hypertrophic remodelling. Eur Heart J 25:1979–1989
Yao JA, Hussain W, Patel P, Peters NS, Boyden PA, Wit AL (2003) Remodeling of gap junctional channel function in epicardial border zone of healing canine infarcts. Circ Res 92:437–443
Efimov IR (2006) Connections, connections, connexins: towards systems biology paradigm of cardiac arrhythmia. J Mol Cell Cardiol 41:949–951
Vinet A, Roberge FA (1994) The dynamics of sustained reentry in a ring model of cardiac tissue. Ann Biomed Eng 22:568–591
Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF (2000) Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol 149:1503–1512
Laird DW, Revel JP (1990) Biochemical and immunochemical analysis of the arrangement of connexin43 in rat heart gap junction membranes. J Cell Sci 97(Pt 1):109–117
Ek-Vitorin JF, King TJ, Heyman NS, Lampe PD, Burt JM (2006) Selectivity of connexin 43 channels is regulated through protein kinase C-dependent phosphorylation. Circ Res 98:1498–1505
Solan JL, Lampe PD (2007) Key connexin 43 phosphorylation events regulate the gap junction life cycle. J Membr Biol 217:35–41
van Kats JP, Danser AH, van Meegen JR, Sassen LM, Verdouw PD, Schalekamp MA (1998) Angiotensin production by the heart: a quantitative study in pigs with the use of radiolabeled angiotensin infusions. Circulation 98:73–81
Donoghue M, Wakimoto H, Maguire CT, Acton S, Hales P, Stagliano N, Fairchild-Huntress V, Xu J, Lorenz JN, Kadambi V et al (2003) Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol 35:1043–1053
Solan JL, Fry MD, TenBroek EM, Lampe PD (2003) Connexin43 phosphorylation at S368 is acute during S and G2/M and in response to protein kinase C activation. J Cell Sci 116:2203–2211
Morita N, Sovari AA, Xie Y, Fishbein MC, Mandel WJ, Garfinkel A, Lin SF, Chen PS, Xie LH, Chen F et al (2009) Increased susceptibility of aged hearts to ventricular fibrillation during oxidative stress. Am J Physiol Heart Circ Physiol 297:H1594–H1605
Iravanian S, Sovari AA, Dudley SC (2010) Renin-angiotensin system modulation of atrioventricular nodal conduction. Heart Rhythm 7:S170
Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI (2001) Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res 88:333–339
Fischer R, Dechend R, Gapelyuk A, Shagdarsuren E, Gruner K, Gruner A, Gratze P, Qadri F, Wellner M, Fiebeler A et al (2007) Angiotensin II-induced sudden arrhythmic death and electrical remodeling. Am J Physiol Heart Circ Physiol 293:H1242–H1253
De Mello WC, Specht P (2006) Chronic blockade of angiotensin II AT1-receptors increased cell-to-cell communication, reduced fibrosis and improved impulse propagation in the failing heart. JRAAS 7:201–205
Polontchouk L, Ebelt B, Jackels M, Dhein S (2002) Chronic effects of endothelin 1 and angiotensin II on gap junctions and intercellular communication in cardiac cells. FASEB J 16:87–89
Inoue N, Ohkusa T, Nao T, Lee JK, Matsumoto T, Hisamatsu Y, Satoh T, Yano M, Yasui K, Kodama I et al (2004) Rapid electrical stimulation of contraction modulates gap junction protein in neonatal rat cultured cardiomyocytes: involvement of mitogen-activated protein kinases and effects of angiotensin II-receptor antagonist. J Am Coll Cardiol 44:914–922
Kleber AG, Rudy Y (2004) Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 84:431–488
Funding
This work was supported by National Institute of Health grants R01 HL085558 (SCD, KEB), R01 HL073753 (SCD), P01 HL058000 (SCD), DK039777 (KEB), American Heart Association (AHA) National Scientist Development Grant (HDX), and AHA postdoctoral fellowship (SI).
Conflicts of interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iravanian, S., Sovari, A.A., Lardin, H.A. et al. Inhibition of renin-angiotensin system (RAS) reduces ventricular tachycardia risk by altering connexin43. J Mol Med 89, 677–687 (2011). https://doi.org/10.1007/s00109-011-0761-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-011-0761-3