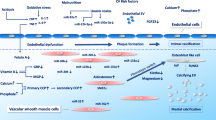
Abstract
It is very well established that purinergic signaling plays a relevant role in vascular physiology and pathophysiology. Recently, a new purinoceptor agonist uridine adenosine tetraphosphate (Up4A) has been identified as a highly potent endothelial-derived contracting factor (EDCF). The purpose of the study was to investigate Up4A's influence on pro-inflammatory mechanisms. An early component of the inflammatory response in atherogenesis is the oxidative stress-induced formation of monocyte chemoattractant protein-1 (MCP-1). Here, we investigated the influence of Up4A on MCP-1 formation and characterized the underlying signaling transduction mechanisms in rat vascular smooth muscle cells (VSMCs). Up4A induced MCP-1 expression and secretion in VSMCs via the activation of P2Y2 in a concentration-dependent manner. MCP-1 formation depends on generation of reactive oxygen species (ROS). To determine whether the predominant source of ROS in the vasculature, the NAD(P)H oxidase (Nox), is involved, we used different approaches. The ROS scavenger, tiron, the Nox inhibitor, apocynin and diphenyl-iodonium, as well as Nox1 knockdown, diminished the Up4A-induced MCP-1 formation. Rac1 activation and p47phox translocation from cytosol to the plasma membrane—both required for assembling and activation of Nox, were stimulated by Up4A. ERK1/2 and p38 activation is essential for the intracellular signal transduction. In summary, Up4A induced Nox1-dependent ROS generation, which further stimulated MCP-1 formation via MAPK phosphorylation in VSMCs. This process requires the activation of the G-protein coupled receptor P2Y2. Therefore, Up4A is not only a potent EDCF but also a potent inductor of pro-inflammatory response in the vascular wall.







Similar content being viewed by others
References
Forstermann U, Munzel T (2006) Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113:1708–1714
Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S (2009) Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br J Pharmacol 157:527–536
Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S (2009) The ageing endothelium, cardiovascular risk and disease in man. Exp Physiol 94:317–321
Vanhoutte PM, Shimokawa H, Tang EH, Feletou M (2009) Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 196:193–222
Jankowski V, Tolle M, Vanholder R, Schonfelder G, van der Giet M, Henning L, Schluter H, Paul M, Zidek W, Jankowski J (2005) Uridine adenosine tetraphosphate: a novel endothelium-derived vasoconstrictive factor. Nat Med 11:223–227
Jankowski V, van der Giet M, Mischak H, Morgan M, Zidek W, Jankowski J (2009) Dinucleoside polyphosphates: strong endogenous agonists of the purinergic system. Br J Pharmacol 157:1142–1153
Jankowski V, Meyer AA, Schlattmann P, Gui Y, Zheng XL, Stamcou I, Radtke K, Tran TN, van der Giet M, Tolle M, Zidek W, Jankowski J (2007) Increased uridine adenosine tetraphosphate concentrations in plasma of juvenile hypertensives. Arterioscler Thromb Vasc Biol 27:1776–1781
Finn AV, Kolodgie FD, Virmani R (2010) Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler Thromb Vasc Biol 30:177–181
Charo IF, Ransohoff RM (2006) The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354:610–621
Boisvert WA (2004) Modulation of atherogenesis by chemokines. Trends Cardiovasc Med 14:161–165
Tolle M, Pawlak A, Schuchardt M, Kawamura A, Tietge UJ, Lorkowski S, Keul P, Assmann G, Chun J, Levkau B, van der Giet M, Nofer JR (2008) HDL-associated lysosphingolipids inhibit NAD(P)H oxidase-dependent monocyte chemoattractant protein-1 production. Arterioscler Thromb Vasc Biol 28:1542–1548
Tolle M, Schuchardt M, Wiedon A, Huang T, Klockel L, Jankowski J, Jankowski V, Zidek W, van der Giet M (2010) Differential effects of uridine adenosine tetraphosphateon purinoceptors in the rat isolated perfused kidney. Br J Pharmacol 161:530–540
Erlinge D, Burnstock G (2008) P2 receptors in cardiovascular regulation and disease. Purinergic signalling 4:1–20
Lassegue B, Griendling KK (2010) NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 30:653–661
Brown DI, Griendling KK (2009) Nox proteins in signal transduction. Free Radic Biol Med 47:1239–1253
Petry A, Weitnauer M, Gorlach A (2010) Receptor activation of NADPH oxidases. Antioxid Redox Signal 13:467–487
Griendling KK, Sorescu D, Ushio-Fukai M (2000) NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86:494–501
Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58:281–341
Stokes L, Surprenant A (2007) Purinergic P2Y2 receptors induce increased MCP-1/CCL2 synthesis and release from rat alveolar and peritoneal macrophages. J Immunol 179:6016–6023
Pacaud P, Malam-Souley R, Loirand G, Desgranges C (1995) ATP raises [Ca2+]i via different P2-receptor subtypes in freshly isolated and cultured aortic myocytes. Am J Physiol 269:H30–H36
von Kugelgen I (2006) Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110:415–432
Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314:1792–1795
Seye CI, Yu N, Gonzalez FA, Erb L, Weisman GA (2004) The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1). J Biol Chem 279:35679–35686
Kukulski F, Ben Yebdri F, Bahrami F, Fausther M, Tremblay A, Sevigny J (2010) Endothelial P2Y2 receptor regulates LPS-induced neutrophil transendothelial migration in vitro. Mol Immunol 47:991–999
Brandes RP, Viedt C, Nguyen K, Beer S, Kreuzer J, Busse R, Gorlach A (2001) Thrombin-induced MCP-1 expression involves activation of the p22phox-containing NADPH oxidase in human vascular smooth muscle cells. Thromb Haemost 85:1104–1110
Ferrari D, Idzko M, Dichmann S, Purlis D, Virchow C, Norgauer J, Chiozzi P, Di Virgilio F, Luttmann W (2000) P2 purinergic receptors of human eosinophils: characterization and coupling to oxygen radical production. FEBS Lett 486:217–224
Lassegue B, Clempus RE (2003) Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285:R277–R297
Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, Harrison DG (2008) Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52:1803–1809
Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK (2008) Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45:1340–1351
Katsuyama M, Ozgur Cevik M, Arakawa N, Kakehi T, Nishinaka T, Iwata K, Ibi M, Matsuno K, Yabe-Nishimura C (2007) Myocyte enhancer factor 2B is involved in the inducible expression of NOX1/NADPH oxidase, a vascular superoxide-producing enzyme. FEBS J 274:5128–5136
Goettsch C, Goettsch W, Muller G, Seebach J, Schnittler HJ, Morawietz H (2009) Nox4 overexpression activates reactive oxygen species and p38 MAPK in human endothelial cells. Biochem Biophys Res Commun 380:355–360
de Groot E, van Leuven SI, Duivenvoorden R, Meuwese MC, Akdim F, Bots ML, Kastelein JJ (2008) Measurement of carotid intima-media thickness to assess progression and regression of atherosclerosis. Nat Clin Pract Cardiovasc Med 5:280–288
Acknowledgments
This work was supported by grants of Deutsche Forschungsgemeinschaft (MvdG, JJ: GI339/7-2); Sonnenfeld-Stiftung (MvdG, MT).
Conflicts of interest
The authors declare no conflict of interests related to this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1.33 MB)
Rights and permissions
About this article
Cite this article
Schuchardt, M., Prüfer, J., Prüfer, N. et al. The endothelium-derived contracting factor uridine adenosine tetraphosphate induces P2Y2-mediated pro-inflammatory signaling by monocyte chemoattractant protein-1 formation. J Mol Med 89, 799–810 (2011). https://doi.org/10.1007/s00109-011-0750-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-011-0750-6