Abstract
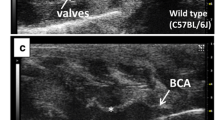
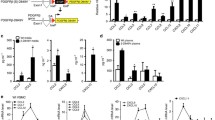
Thromboxane A2 (TXA2) is a potent prothrombotic and immune modulating lipid mediator, which is implicated in cardiovascular diseases, in particular, atherosclerotic lesion development and thrombogenicity. Here, we tested the hypothesis that thromboxane synthase (TXAS), the obligate enzyme required to synthesize TXA2, is expressed within the human atherosclerotic lesion, thus potentially contributing to TXA2 synthesis and disease development. In an animal study, different atherosclerosis-prone mouse strains were investigated and compared with control mice. In a patient study (n = 134), endarterectomies of carotid atherosclerotic lesions were compared with non-atherosclerotic arteries (n = 11). Expression of TXAS was evaluated by real-time quantitative reverse transcription PCR and immunohistochemistry. TXAS mRNA expression was increased within the vascular wall in mouse models of atherosclerosis with advanced lesions. In humans, TXAS was expressed in the atherosclerotic lesion, associated with increased inflammatory cells, in particular M2 polarized macrophages, and increased in atherosclerotic lesions of patients with recent symptoms of thrombotic events. Production of TXA2 by plaque tissue, verified by gas chromatography–mass spectrometry, increased after addition of arachidonic acid or lipopolysaccharide, and was inhibited by the TXAS inhibitor furegrelate. The findings suggest that intraplaque TXA2 generation may contribute to the development of atherosclerosis and its thrombotic complications in humans.





Similar content being viewed by others
References
Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352:1685–1695
Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C et al (2003) From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part II. Circulation 108:1772–1778
Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P et al (2003) From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation 108:1664–1672
Hamberg M, Svensson J, Samuelsson B (1975) Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci USA 72:2994–2998
Oates JA, FitzGerald GA, Branch RA, Jackson EK, Knapp HR, Roberts LJ 2nd (1988) Clinical implications of prostaglandin and thromboxane A2 formation (2). N Engl J Med 319:761–767
Oates JA, FitzGerald GA, Branch RA, Jackson EK, Knapp HR, Roberts LJ 2nd (1988) Clinical implications of prostaglandin and thromboxane A2 formation (1). N Engl J Med 319:689–698
Patrono C, Coller B, FitzGerald GA, Hirsh J, Roth G (2004) Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126:234S–264S
Shen RF, Tai HH (1998) Thromboxanes: synthase and receptors. J Biomed Sci 5:153–172
Yu IS, Lin SR, Huang CC, Tseng HY, Huang PH, Shi GY, Wu HL, Tang CL, Chu PH, Wang LH, Wu KK, Lin SW (2004) TXAS-deleted mice exhibit normal thrombopoiesis, defective hemostasis, and resistance to arachidonate-induced death. Blood 104:135–142
Samuelsson B, Hamberg M, Malmsten C, Svensson J (1976) The role of prostaglandin endoperoxides and thromboxanes in platelet aggregation. Adv Prostaglandin Thromboxane Res 2:737–746
Takayama K, Yuhki K, Ono K, Fujino T, Hara A, Yamada T, Kuriyama S, Karibe H, Okada Y, Takahata O et al (2005) Thromboxane A2 and prostaglandin F2alpha mediate inflammatory tachycardia. Nat Med 11:562–566
Needleman P, Moncada S, Bunting S, Vane JR, Hamberg M, Samuelsson B (1976) Identification of an enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature 261:558–560
Fitzgerald GA (2004) Coxibs and cardiovascular disease. N Engl J Med 351:1709–1711
Mehta J, Roberts A (1983) Human vascular tissues produce thromboxane as well as prostacyclin. Am J Physiol 244:R839–R844
Pawlowski NA, Kaplan G, Hamill AL, Cohn ZA, Scott WA (1983) Arachidonic acid metabolism by human monocytes. Studies with platelet-depleted cultures. J Exp Med 158:393–412
Kennedy MS, Stobo JD, Goldyne ME (1980) In vitro synthesis of prostaglandins and related lipids by populations of human peripheral blood mononuclear cells. Prostaglandins 20:135–145
Neri Serneri GG, Gensini GF, Poggesi L, Modesti PA, Rostagno C, Boddi M, Gori AM, Martini F, Ieri A, Margheri M, Abbate R (1994) The role of extraplatelet thromboxane A2 in unstable angina investigated with a dual thromboxane A2 inhibitor: importance of activated monocytes. Coron Artery Dis 5:137–145
Kobayashi T, Tahara Y, Matsumoto M, Iguchi M, Sano H, Murayama T, Arai H, Oida H, Yurugi-Kobayashi T, Yamashita JK et al (2004) Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J Clin Invest 114:784–794
Cayatte AJ, Du Y, Oliver-Krasinski J, Lavielle G, Verbeuren TJ, Cohen RA (2000) The thromboxane receptor antagonist S18886 but not aspirin inhibits atherogenesis in apoE-deficient mice: evidence that eicosanoids other than thromboxane contribute to atherosclerosis. Arterioscler Thromb Vasc Biol 20:1724–1728
Cyrus T, Yao Y, Ding T, Dogne JM, Pratico D (2007) Thromboxane receptor blockade improves the antiatherogenic effect of thromboxane A2 suppression in LDLR KO mice. Blood 109:3291–3296
Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C (2005) Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med 353:2373–2383
Caligiuri G, Levy B, Pernow J, Thoren P, Hansson GK (1999) Myocardial infarction mediated by endothelin receptor signaling in hypercholesterolemic mice. Proc Natl Acad Sci USA 96:6920–6924
Martinez FO, Sica A, Mantovani A, Locati M (2008) Macrophage activation and polarization. Front Biosci 13:453–461
Back M, Qiu H, Haeggstrom JZ, Sakata K (2004) Leukotriene B4 is an indirectly acting vasoconstrictor in guinea pig aorta via an inducible type of BLT receptor. Am J Physiol Heart Circ Physiol 287:H419–H424
Redgrave JN, Lovett JK, Gallagher PJ, Rothwell PM (2006) Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: the Oxford Plaque Study. Circulation 113:2320–2328
Kleindorfer D, Panagos P, Pancioli A, Khoury J, Kissela B, Woo D, Schneider A, Alwell K, Jauch E, Miller R, Moomaw C, Shukla R, Broderick JP (2005) Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke 36:720–723
Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ (2002) Expression of Toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation 105:1158–1161
Hwang D (2001) Modulation of the expression of cyclooxygenase-2 by fatty acids mediated through Toll-like receptor 4-derived signaling pathways. Faseb J 15:2556–2564
Back M, Bu DX, Branstrom R, Sheikine Y, Yan ZQ, Hansson GK (2005) Leukotriene B4 signaling through NF-kappaB-dependent BLT1 receptors on vascular smooth muscle cells in atherosclerosis and intimal hyperplasia. Proc Natl Acad Sci USA 102:17501–17506
Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G (2007) PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 6:137–143
Daniel TO, Liu H, Morrow JD, Crews BC, Marnett LJ (1999) Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogenesis. Cancer Res 59:4574–4577
Ashton AW, Ware JA (2004) Thromboxane A2 receptor signaling inhibits vascular endothelial growth factor-induced endothelial cell differentiation and migration. Circ Res 95:372–379
Ashton AW, Cheng Y, Helisch A, Ware JA (2004) Thromboxane A2 receptor agonists antagonize the proangiogenic effects of fibroblast growth factor-2: role of receptor internalization, thrombospondin-1, and alpha(v)beta3. Circ Res 94:735–742
Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo KM, Gillies S, Javaherian K, Folkman J (2003) Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci USA 100:4736–4741
Kabashima K, Murata T, Tanaka H, Matsuoka T, Sakata D, Yoshida N, Katagiri K, Kinashi T, Tanaka T, Miyasaka M, Nagai H, Ushikubi F, Narumiya S (2003) Thromboxane A2 modulates interaction of dendritic cells and T cells and regulates acquired immunity. Nat Immunol 4:694–701
Ishizuka T, Matsumura K, Matsui T, Takase B, Kurita A (2003) Ramatroban, a thromboxane A2 receptor antagonist, prevents macrophage accumulation and neointimal formation after balloon arterial injury in cholesterol-fed rabbits. J Cardiovasc Pharmacol 41:571–578
Egan KM, Wang M, Fries S, Lucitt MB, Zukas AM, Pure E, Lawson JA, FitzGerald GA (2005) Cyclooxygenases, thromboxane, and atherosclerosis: plaque destabilization by cyclooxygenase-2 inhibition combined with thromboxane receptor antagonism. Circulation 111:334–342
Acknowledgements
These studies were supported by the Linneus Center for Research on Inflammation and Cardiovascular Disease of the Swedish Research Council and by grants from AFA health insurance company foundation, the Swedish Heart-Lung Foundation, Söderberg Foundation, The Swedish Research Council (6816-10350, 71X-14121, and 20854), the Konung Gustav V:s 80 årsfond, and EC FP6 (LSHM-CT-2004-005033). The report reflects only the author's views, and the European Commission is not liable for any use that may be made of the information herein. The work of A. Gabriele was supported by a European Union Marie Curie fellowship. The work of G. Paulsson-Berne was supported by the Swedish Research Council.
Disclosures
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gabrielsen, A., Qiu, H., Bäck, M. et al. Thromboxane synthase expression and thromboxane A2 production in the atherosclerotic lesion. J Mol Med 88, 795–806 (2010). https://doi.org/10.1007/s00109-010-0621-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-010-0621-6