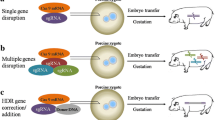
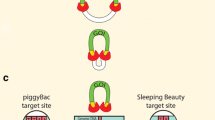
Abstract
The translation of novel discoveries from basic research to clinical application is a long, often inefficient, and thus costly process. Accordingly, the process of drug development requires optimization both for economic and for ethical reasons, in order to provide patients with appropriate treatments in a reasonable time frame. Consequently, “Translational Medicine” became a top priority in national and international roadmaps of human health research. Appropriate animal models for the evaluation of efficacy and safety of new drugs or therapeutic concepts are critical for the success of translational research. In this context rodent models are most widely used. At present, transgenic pigs are increasingly being established as large animal models for selected human diseases. The first pig whole genome sequence and many other genomic resources will be available in the near future. Importantly, efficient and precise techniques for the genetic modification of pigs have been established, facilitating the generation of tailored disease models. This article provides an overview of the current techniques for genetic modification of pigs and the transgenic pig models established for neurodegenerative diseases, cardiovascular diseases, cystic fibrosis, and diabetes mellitus.




Similar content being viewed by others
References
Wehling M (2008) Translational medicine: science or wishful thinking? J Transl Med 6:31
Phillips KA, Van Bebber S, Issa AM (2006) Diagnostics and biomarker development: priming the pipeline. Nat Rev Drug Discov 5:463–469
Lunney JK (2007) Advances in swine biomedical model genomics. Int J Biol Sci 3:179–184
Wolf E, Schernthaner W, Zakhartchenko V, Prelle K, Stojkovic M, Brem G (2000) Transgenic technology in farm animals—progress and perspectives. Exp Physiol 85:615–625
Rehbinder C, Baneux P, Forbes D, van Herck H, Nicklas W, Rugaya Z, Winkler G (1998) FELASA recommendations for the health monitoring of breeding colonies and experimental units of cats, dogs and pigs. Report of the Federation of European Laboratory Animal Science Associations (FELASA) Working Group on Animal Health. Lab Anim 32:1–17
Mezrich JD, Haller GW, Arn JS, Houser SL, Madsen JC, Sachs DH (2003) Histocompatible miniature swine: an inbred large-animal model. Transplantation 75:904–907
Wang X, Ou J, Huang L, Nishihara M, Li J, Manabe N, Zhang Y (2006) Genetic characteristics of inbred Wuzhishan miniature pigs, a native Chinese breed. J Reprod Dev 52:639–643
Chen K, Baxter T, Muir WM, Groenen MA, Schook LB (2007) Genetic resources, genome mapping and evolutionary genomics of the pig (Sus scrofa). Int J Biol Sci 3:153–165
Wernersson R, Schierup MH, Jorgensen FG, Gorodkin J, Panitz F, Staerfeldt HH, Christensen OF, Mailund T, Hornshoj H, Klein A et al (2005) Pigs in sequence space: a 0.66X coverage pig genome survey based on shotgun sequencing. BMC Genomics 6:70
Jorgensen FG, Hobolth A, Hornshoj H, Bendixen C, Fredholm M, Schierup MH (2005) Comparative analysis of protein coding sequences from human, mouse and the domesticated pig. BMC Biol 3:2
Ramos AM, Crooijmans RP, Affara NA, Amaral AJ, Archibald AL, Beever JE, Bendixen C, Churcher C, Clark R, Dehais P et al (2009) Design of a high density SNP genotyping assay in the pig using SNPs identified and characterized by next generation sequencing technology. PLoS ONE 4:e6524
Gorodkin J, Cirera S, Hedegaard J, Gilchrist MJ, Panitz F, Jorgensen C, Scheibye-Knudsen K, Arvin T, Lumholdt S, Sawera M et al (2007) Porcine transcriptome analysis based on 97 non-normalized cDNA libraries and assembly of 1, 021, 891 expressed sequence tags. Genome Biol 8:R45
Brem G, Brenig B, Goodman HM, Selden RC, Graf F, Kruff B, Springmann K, Hondele J, Meyer J, Winnacker EL et al (1985) Production of transgenic mice, rabbits and pigs by microinjection into pronuclei. Zuchthygiene 20:251–252
Hammer RE, Pursel VG, Rexroad CE Jr, Wall RJ, Bolt DJ, Ebert KM, Palmiter RD, Brinster RL (1985) Production of transgenic rabbits, sheep and pigs by microinjection. Nature 315:680–683
Lavitrano M, Busnelli M, Cerrito MG, Giovannoni R, Manzini S, Vargiolu A (2006) Sperm-mediated gene transfer. Reprod Fertil Dev 18:19–23
Lavitrano M, Forni M, Bacci ML, Di Stefano C, Varzi V, Wang H, Seren E (2003) Sperm mediated gene transfer in pig: selection of donor boars and optimization of DNA uptake. Mol Reprod Dev 64:284–291
Chang K, Qian J, Jiang M, Liu YH, Wu MC, Chen CD, Lai CK, Lo HL, Hsiao CT, Brown L et al (2002) Effective generation of transgenic pigs and mice by linker based sperm-mediated gene transfer. BMC Biotechnol 2:5
Kurome M, Ueda H, Tomii R, Naruse K, Nagashima H (2006) Production of transgenic-clone pigs by the combination of ICSI-mediated gene transfer with somatic cell nuclear transfer. Transgenic Res 15:229–240
Pfeifer A (2004) Lentiviral transgenesis. Transgenic Res 13:513–522
Hofmann A, Kessler B, Ewerling S, Weppert M, Vogg B, Ludwig H, Stojkovic M, Boelhauve M, Brem G, Wolf E et al (2003) Efficient transgenesis in farm animals by lentiviral vectors. EMBO Rep 4:1054–1060
Whitelaw CB, Radcliffe PA, Ritchie WA, Carlisle A, Ellard FM, Pena RN, Rowe J, Clark AJ, King TJ, Mitrophanous KA (2004) Efficient generation of transgenic pigs using equine infectious anaemia virus (EIAV) derived vector. FEBS Lett 571:233–236
Hofmann A, Kessler B, Ewerling S, Kabermann A, Brem G, Wolf E, Pfeifer A (2006) Epigenetic regulation of lentiviral transgene vectors in a large animal model. Molec Ther 13:59–66
Betthauser J, Forsberg E, Augenstein M, Childs L, Eilertsen K, Enos J, Forsythe T, Golueke P, Jurgella G, Koppang R et al (2000) Production of cloned pigs from in vitro systems. Nat Biotechnol 18:1055–1059
Onishi A, Iwamoto M, Akita T, Mikawa S, Takeda K, Awata T, Hanada H, Perry AC (2000) Pig cloning by microinjection of fetal fibroblast nuclei. Science 289:1188–1190
Polejaeva IA, Chen SH, Vaught TD, Page RL, Mullins J, Ball S, Dai Y, Boone J, Walker S, Ayares DL et al (2000) Cloned pigs produced by nuclear transfer from adult somatic cells. Nature 407:86–90
Du Y, Kragh PM, Zhang Y, Li J, Schmidt M, Bogh IB, Zhang X, Purup S, Jorgensen AL, Pedersen AM et al (2007) Piglets born from handmade cloning, an innovative cloning method without micromanipulation. Theriogenology 68:1104–1110
Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN et al (2002) Production of alpha-1, 3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295:1089–1092
Rogers CS, Hao Y, Rokhlina T, Samuel M, Stoltz DA, Li Y, Petroff E, Vermeer DW, Kabel AC, Yan Z et al (2008) Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest 118:1571–1577
Shi W, Zakhartchenko V, Wolf E (2003) Epigenetic reprogramming in mammalian nuclear transfer. Differentiation 71:91–113
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Madsen LB, Thomsen B, Solvsten CA, Bendixen C, Fredholm M, Jorgensen AL, Nielsen AL (2007) Identification of the porcine homologous of human disease causing trinucleotide repeat sequences. Neurogenetics 8:207–218
Kragh PM, Nielsen AL, Li J, Du Y, Lin L, Schmidt M, Bogh IB, Holm IE, Jakobsen JE, Johansen MG et al (2009) Hemizygous minipigs produced by random gene insertion and handmade cloning express the Alzheimer’s disease-causing dominant mutation APPsw. Transgenic Res 18:545–558
Matsuyama N, Hadano S, Onoe K, Osuga H, Showguchi-Miyata J, Gondo Y, Ikeda JE (2000) Identification and characterization of the miniature pig Huntington’s disease gene homolog: evidence for conservation and polymorphism in the CAG triplet repeat. Genomics 69:72–85
Uchida M, Shimatsu Y, Onoe K, Matsuyama N, Niki R, Ikeda JE, Imai H (2001) Production of transgenic miniature pigs by pronuclear microinjection. Transgenic Res 10:577–582
Gregory-Evans K, Weleber RG (1997) An eye for an eye: new models of genetic ocular disease. Nat Biotechnol 15:947–948
Petters RM, Alexander CA, Wells KD, Collins EB, Sommer JR, Blanton MR, Rojas G, Hao Y, Flowers WL, Banin E et al (1997) Genetically engineered large animal model for studying cone photoreceptor survival and degeneration in retinitis pigmentosa. Nat Biotechnol 15:965–970
Kraft TW, Allen D, Petters RM, Hao Y, Peng YW, Wong F (2005) Altered light responses of single rod photoreceptors in transgenic pigs expressing P347L or P347S rhodopsin. Mol Vis 11:1246–1256
Lorson MA, Spate LD, Prather RS, Lorson CL (2008) Identification and characterization of the porcine (Sus scrofa) survival motor neuron (SMN1) gene: an animal model for therapeutic studies. Dev Dyn 237:2268–2278
Huang PL (2009) eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol Metab 20:295–302
Hao YH, Yong HY, Murphy CN, Wax D, Samuel M, Rieke A, Lai L, Liu Z, Durtschi DC, Welbern VR et al (2006) Production of endothelial nitric oxide synthase (eNOS) over-expressing piglets. Transgenic Res 15:739–750
Prather RS (2007) Targeted genetic modification: xenotransplantation and beyond. Cloning Stem Cells 9:17–20
Guilbault C, Saeed Z, Downey GP, Radzioch D (2007) Cystic fibrosis mouse models. Am J Respir Cell Mol Biol 36:1–7
Rogers CS, Abraham WM, Brogden KA, Engelhardt JF, Fisher JT, McCray PB Jr, McLennan G, Meyerholz DK, Namati E, Ostedgaard LS et al (2008) The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 295:L240–L263
Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA et al (2008) Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 321:1837–1841
Plum L, Wunderlich FT, Baudler S, Krone W, Bruning JC (2005) Transgenic and knockout mice in diabetes research: novel insights into pathophysiology, limitations, and perspectives. Physiology 20:152–161
Aigner B, Rathkolb B, Herbach N, Hrabé de Angelis M, Wanke R, Wolf E (2008) Diabetes models by screen for hyperglycemia in phenotype-driven ENU mouse mutagenesis projects. Am J Physiol Endocrinol Metab 294:E232–E240
Prentki M, Nolan CJ (2006) Islet beta cell failure in type 2 diabetes. J Clin Invest 116:1802–1812
Baggio LL, Drucker DJ (2007) Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157
Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W (1993) Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 91:301–307
Lovshin JA, Drucker DJ (2009) Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5:262–269
Nauck MA, Baller B, Meier JJ (2004) Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes 53(Suppl 3):S190–S196
Saxena R, Hivert MF, Langenberg C, Tanaka T, Pankow JS, Vollenweider P, Lyssenko V, Bouatia-Naji N, Dupuis J, Jackson AU et al (2010) Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet 42:142–148
Miyawaki K, Yamada Y, Yano H, Niwa H, Ban N, Ihara Y, Kubota A, Fujimoto S, Kajikawa M, Kuroe A et al (1999) Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci U S A 96:14843–14847
Hansotia T, Drucker DJ (2005) GIP and GLP-1 as incretin hormones: lessons from single and double incretin receptor knockout mice. Regul Pept 128:125–134
Herbach N, Goeke B, Schneider M, Hermanns W, Wolf E, Wanke R (2005) Overexpression of a dominant negative GIP receptor in transgenic mice results in disturbed postnatal pancreatic islet and beta-cell development. Regul Pept 125:103–117
Renner S, Fehlings C, Herbach N, Hofmann A, von Waldthausen DC, Kessler B, Ulrichs K, Chodnevskaja I, Moskalenko V, Amselgruber W et al (2010) Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired GIP function. Diabetes. doi:10.2337/db09-0519
Gotthardt M, Lalyko G, Eerd-Vismale J, Keil B, Schurrat T, Hower M, Laverman P, Behr TM, Boerman OC, Goke B et al (2006) A new technique for in vivo imaging of specific GLP-1 binding sites: first results in small rodents. Regul Pept 137:162–167
Herbach N, Rathkolb B, Kemter E, Pichl L, Klaften M, Hrabé de Angelis M, Halban PA, Wolf E, Aigner B, Wanke R (2007) Dominant-negative effects of a novel mutated Ins2 allele causes early-onset diabetes and severe beta-cell loss in Munich Ins2C95S mutant mice. Diabetes 56:1268–1276
Umeyama K, Watanabe M, Saito H, Kurome M, Tohi S, Matsunari H, Miki K, Nagashima H (2009) Dominant-negative mutant hepatocyte nuclear factor 1alpha induces diabetes in transgenic-cloned pigs. Transgenic Res 18:697–706
Kues WA, Schwinzer R, Wirth D, Verhoeyen E, Lemme E, Herrmann D, Barg-Kues B, Hauser H, Wonigeit K, Niemann H (2006) Epigenetic silencing and tissue independent expression of a novel tetracycline inducible system in double-transgenic pigs. FASEB J 20:1200–1202
Li L, Pang D, Wang T, Li Z, Chen L, Zhang M, Song N, Nie D, Chen Z, Lai L et al (2009) Production of a reporter transgenic pig for monitoring Cre recombinase activity. Biochem Biophys Res Commun 382:232–235
Manzini S, Vargiolu A, Stehle IM, Bacci ML, Cerrito MG, Giovannoni R, Zannoni A, Bianco MR, Forni M, Donini P et al (2006) Genetically modified pigs produced with a nonviral episomal vector. Proc Natl Acad Sci U S A 103:17672–17677
Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X et al (2009) Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325:433
Bertolini LR, Bertolini M, Maga EA, Madden KR, Murray JD (2009) Increased gene targeting in Ku70 and Xrcc4 transiently deficient human somatic cells. Mol Biotechnol 41:106–114
Yan Z, Sun X, Engelhardt JF (2009) Progress and prospects: techniques for site-directed mutagenesis in animal models. Gene Ther 16:581–588
McCalla-Martin AC, Chen X, Linder KE, Estrada JL, Piedrahita JA (2010) Varying phenotypes in swine versus murine transgenic models constitutively expressing the same human Sonic hedgehog transcriptional activator, K5-HGLI2DeltaN. Transgenic Res. doi:10.1007/s11248-010-9362-0
Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I et al (2009) Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A 106:14022–14027
Esteban MA, Xu J, Yang J, Peng M, Qin D, Li W, Jiang Z, Chen J, Deng K, Zhong M et al (2009) Generation of induced pluripotent stem cell lines from Tibetan miniature pig. J Biol Chem 284:17634–17640
Ezashi T, Telugu BP, Alexenko AP, Sachdev S, Sinha S, Roberts RM (2009) Derivation of induced pluripotent stem cells from pig somatic cells. Proc Natl Acad Sci U S A 106:10993–10998
Wu Z, Chen J, Ren J, Bao L, Liao J, Cui C, Rao L, Li H, Gu Y, Dai H et al (2009) Generation of pig induced pluripotent stem cells with a drug-inducible system. J Mol Cell Biol 1:46–54
Rho GJ, Kumar BM, Balasubramanian SS (2009) Porcine mesenchymal stem cells—current technological status and future perspective. Front Biosci 14:3942–3961
Matsunari H, Nagashima H (2009) Application of genetically modified and cloned pigs in translational research. J Reprod Dev 55:225–230
Acknowledgments
Our projects involving the development of large animal models for translational research were/are supported by the Deutsche Forschungsgemeinschaft (FOR535, FOR793, FOR1041, GRK1029), the Bundesministerium für Bildung und Forschung (MoBiMed), the Mukoviszidose e.V., and the Bayerische Forschungsstiftung (492/02, FORZEBRA).
Disclosure of potential conflict of interests
The authors declare no conflict of interests related to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aigner, B., Renner, S., Kessler, B. et al. Transgenic pigs as models for translational biomedical research. J Mol Med 88, 653–664 (2010). https://doi.org/10.1007/s00109-010-0610-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-010-0610-9