Abstract
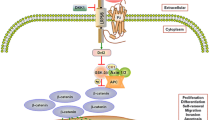
In contrast to the transforming members of the Wnt family, shown to be upregulated in many cancers, the role of Wnt 5a is still controversial. While it has been attributed a tumour suppressor function in some malignancies, there is increasing evidence of promigratory and proinvasive effects in others, mediated predominantly through the planar cell polarity pathway and activation of protein kinase C. Obviously, the outcome of an individual Wnt 5a signal is dependent on a multitude of variables, ranging from availability of receptors, downstream effectors, and inhibitors to external influences coming from the tumour microenvironment and the extracellular matrix.


Similar content being viewed by others
References
Clevers H (2006) Wnt/β-catenin signaling in development and disease. Cell 127:469–480
Nusse R (2005) Wnt signaling in disease and development. Cell Res 15:28–32
Wong GT, Gavin BJ, McMahon AP (1994) Differential transformation of mammary epithelial cells by Wnt genes. Mol Cell Biol 14:6278–6286
Olson DJ, Papkoff J (1994) Regulated expression of Wnt family members during proliferation of C57mg mammary cells. Cell Growth Differ 5:197–206
Reya T, Clevers H (2005) Wnt signalling in stem cells and cancer. Nature 434:843–850
Fodde R, Brabletz T (2007) Wnt/beta-catenin signaling in cancer stemness and malignant behaviour. Curr Opin Cell Biol 19:150–158
Wallingford JB, Habas R (2005) The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development 132:4421–4436
Wnt homepage (2007) http://www.stanford.edu/~rnusse/wntwindow.html
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512
Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T (1999) β-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol 155:1033–1038
Gradl D, Kühl M, Wedlich D (1999) The Wnt/Wg signal transducer beta-catenin controls fibronectin expression. Mol Cell Biol 19:5576–5587
Slusarski DC, Corces VG, Moon RT (1997) Interaction of Wnt and a frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 390:410–413
Pandur P, Maurus D, Kühl M (2002) Increasingly complex: new players enter the Wnt signaling network. Bioessays 24:881–884
Seifert JR, Mlodzik M (2007) Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet 8:126–138
Jones C, Chen P (2007) Planar cell polarity signaling in vertebrates. Bioessays 29:120–132
Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, Dai X, Chen P (2007) Wnt 5a functions in planar cell polarity regulation in mice. Dev Biol 306:121–133
Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y (2003) Wnt 5a inhibits the canonical Wnt pathway by promoting GSK-3-independent . b. -catenin degradation. J Cell Biol 162:899–908
Westfall TA, Brimeyer R, Twedt J, Gladon J, Olberding A, Furutani-Seiki M, Slusarski DC (2003) Wnt 5a/pipetail fundctions in vertebrate axis formation as a negative regulator of Wnt/beta-catenin signaling. J Cell Biol 162:889–898
Oishi I, Suzuki H, Onishi N, Takada R, Kanai S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y (2003) The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 8:645–654
Schambony A, Wedlich D (2007) Wnt-5a/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell 5:779–792
Mikels AJ, Nusse R (2006) Purified Wnt5a protein activates or inhibits . b. -catenin-TCF signaling depending on receptor context. PLoS Biol 4:570–582
Chen RH, Ding VW, McCormick F (2000) Wnt signaling to . b. -catenin involves two interactive components, glycogen synthase kinase-3b inhibition and activation of protein kinase C. J Biol Chem 275:17894–17899
Nateri AS, Spencer-Dene B, Behrens A (2005) Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature 437:281–285
He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H (1997) A member of the frizzled protein family mediating axis induction by Wnt 5a. Science 275:1652–1654
Kawano Y, Kypta R (2003) Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116:2627–2634
Niehrs C (2006) Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25:7469–7481
Burbelo P, Wellstein A, Pestell RG (2004) Altered Rho GTPase signaling pathways in breast cancer cells. Breast Cancer Res Treat 84:43–48
Liu N, Zhang G, Bi F, Pan Y, Xue Y, Shi Y, Yao L, Zhao L, Zheng Y, Fan D (2007) RhoC is essential for the metastasis of gastric cancer. J Mol Med 85:1149–1156
Yamaguchi TP, Bradley A, McMahon AP, Jones S (1999) A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126:1211–1223
Li C, Xiao J, Hormi K, Borok Z, Minoo P (2002) Wnt5a participates in distal lung morphogenesis. Dev Biol 248:68–81
Kouros-Mehr H, Werb Z (2006) Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn 235:3404–3412
Behbod F, Xian W, Shaw CA, Hilsenbeck SG, Tsimelzon A, Rosen JM (2006) Transcriptional profiling of mammary gland side population cells. Stem Cells 24:1065–1074
Balic M, Lin Y, Young L, Haws D, Guiliano A, McNamara G, Datar RH, Cote RJ (2006) Most early disseminated cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res 12:5615–5621
Mericskay M, Kitajewski J, Sassoon D (2004) Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development 131:2061–2072
Sonderegger S, Husslein H, Leisser C, Knöfler M (2007) Complex expression pattern of Wnt ligands and frizzled receptors in human placenta and its trophoblast subtypes. Placenta 28(Suppl A):97–102
Hayashi K, Burghardt RC, Bazer FW, Spencer TE (2007) WNTs in the ovine uterus: potential regulation of periimplantation ovine conceptus development. Endocrinology 48:3496–3506
Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, Trumper L, Binder C (2006) Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci 103:5454–549
Le Floch N, Rivat C, De Wever O, Bruyneel E, Mareel M, Dale T, Gespach C (2005) The proinvasive activity of Wnt-2 is mediated through a noncanonical Wnt pathway coupled to GSK-3beta and c-Jun/AP-1 signaling. FASEB J 19:144–146
Balkwill F (2006) TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev 25:409–416
Prieve MG, Moon RT (2003) Stromelysin-1 and mesothelin are differentially regulated by Wnt-5a and Wnt-1 in C57mg mouse mammary epithelial cells. BMC Dev Biol 3:2
Maskauchuan TN, Agalliu D, Vorontchikhina M, Ahn A, Parmalee NL, Li CM, Khoo A, Tycko B, Brown AM, Kitajewski J (2006) Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol Biol Cell 17:5163–5172
Fathke C, Wilson L, Shah K, Kim B, Hocking A, Moon RT, Isik F (2006) Wnt signaling induces epithelial differentiation during cutaneous wound healing. BMC Cell Biol 7:4
Bui TD, Zhang L, Rees MC, Bicknell R, Harris AL (1997) Expression and hormone regulation of Wnt2, 3, 4, 5a, 7a, 7b and 10b in normal human endometrium and endometrial carcinoma. Br J Cancer 75:1131–1136
Blanc E, Goldschneider D, Douc-Rasy S, Benard J, Raguenez G (2005) Wnt-5a gene expression in malignant human neuroblasts. Cancer Lett 228:117–123
Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A, Gerstein R, Jurecic R, Jones SN (2003) Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoetic tissue. Cancer Cell 4:349–360
Kremenevskaja N, von Wasielewski R, Rao AS, Schofl C, Andersson T, Brabant G (2005) Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene 24:2144–54
Dejmek J, Dejmek A, Safholm A, Sjolander A, Andersson T (2005) Wnt-5a protein expression in primary dukes B colon cancers identifies a subgroup of patients with good prognosis. Cancer Res 65:9142–9146
Jönsson M, Dejmek J, Bendahl PO, Andersson T (2002) Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res 62:409–416
Dejmek J, Leandersson K, Manjer J, Bjartell A, Emdin SO, Vogel WF, Landberg G, Andersson T (2005) Expression and signaling activity of Wnt-5a/discoidin domain receptor-1 and Syk plays distinct but decisive roles in breast cancer patient survival. Clin Cancer Res 11:520–528
Iozzo RV, Eichstetter I, Danielson KG (1995) Aberrant expression of the growth factor Wnt-5A in human malignancy. Cancer Res 55:3495–3499
Lejeune S, Huguet EL, Hamby A, Poulsom R, Harris AL (1995) Wnt5a cloning, expression, and up-regulation in human primary breast cancers. Clin Cancer Res 1:215–222
Fernandez-Cobo M, Zammarchi F, Mandeli J, Holland JF, Pogo BG (2007) Expression of Wnt5A and Wnt10B in non-immortalized breast cancer cells. Oncol Rep 17:903–907
Zeng ZY, Zhou YH, Zhang WL, Xiong W, Fan SQ, Li XL, Luo XM, Wu MH, Yang YX, Huang C, Cao L, Tang K, Qian J, Shen SR, Li GY (2007) Gene expression profiling of nasopharyngeal carcinoma reveals the abnormally regulated Wnt signaling pathway. Hum Pathol 38:120–133
Ripka S, Konig A, Buchholz M, Wagner M, Sipos B, Kloppel G, Downward J, Gress T, Michl P (2007) WNT5A-target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis 6:1178–1187
Taki M, Kamata N, Yokoyama K, Fujimoto R, Tsutsumi S, Nagayama M (2003) Down-regulation of Wnt-4 and up-regulation of Wnt-5a expression by epithelial-mesenchymal transition in human squamous carcinoma cells. Cancer Sci 94:593–597
Huang CL, Liu D, Nakano J, Ishikawa S, Kontani K, Yokomise H, Ueno M (2005) Wnt5a expression is associated with the tumor proliferation and the stromal vascular endothelial growth factor - an expression in non-small-cell lung cancer. J Clin Oncol 23:8765–8773
Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A (2006) Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 66:10439–10448
Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben-Dor A, Sampas N, Dougherty E, Wang E, Marincola F, Gooden C, Lueders J, Glatfelter A, Pollock P, Carpten J, Gillanders E, Leja D, Dietrich K, Beaudry C, Berens M, Alberts D, Sondak V (2000) Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 406:536–540
Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM (2002) Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 1:279–288
Smith K, Bui TD, Poulsom R, Kaklamanis L, Williams G, Harris AL (1999) Up-regulation of macrophage wnt gene expression in adenoma-carcinoma progression of human colorectal cancer. Br J Cancer 81:496–502
Olson DJ, Gibo DM (1998) Antisense wnt-5a mimics wnt1-mediated C57MG mammary epithelial cell transformation. Exp Cell Res 241:134–141
Jönsson M, Andersson T (2001) Repression of Wnt-5a impairs DDR1 phosphorylation and modifies adhesion and migration of mammary cells. J Cell Sci. 2001 114:2043–2053
Säfholm A, Leandersson K, Dejmek J, Nielsen CK, Villoutreix BO, Andersson T (2006) A formylated hexapeptide ligand mimics the ability of Wnt-5a to impair migration of human breast epithelial cells. J Biol Chem 281:2740–49
Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y, Takada S, Kikuchi A, Minami Y (2006) Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol 175:555–562
Dissanayake SK, Wade M, Johnson CE, O'Connell MP, Leotlela PD, French AD, Shah KV, Hewitt KJ, Rosenthal DT, Indig FE, Jiang Y, Nickoloff BJ, Taub DD, Trent JM, Moon RT, Bittner M, Weeraratna AT (2007) The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem 282:17259–17271
Schenk S, Quaranta V (2003) Tales from the crypt[ic] sites of the extracellular matrix. Trends Cell Biol 13:366–375
Mareel M, Leroy A (2003) Clinical, cellular and molecular aspects of cancer invasion. Physiol Rev 83:337–376
Mikels AJ, Nusse R (2006) Wnts as ligands: processing, secretion and reception. Oncogene 25:7461–7468
Ai X, Do AT, Lozynska O, Kusche-Gullberg M, Lindahl U, Emerson CP (2003) QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J Cell Biol 162:341–351
Kurayoshi M, Yamamoto H, Izumi S, Kikuchi A (2007) Post-translational palmitoylation and glycosylation of Wnt-5a are necassary for its signaling. Biochemical J 402:515–523
Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, Kirchner T (2005) Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and ß-catenin. Cells Tissues Organs 179:56–65
Brabletz T, Jung A, Hermann K, Gunther K, Hohenberger W, Kirchner T (1998) Nuclear overexpression of the oncoprotein beta-catenin in colorectal cancer is localized predominantly at the invasive front. Pathol Res Pract 194:701–704
Gregorieff A, Pinto D, Begthel H, Destrée O, Kielman M, Clevers H (2005) Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology 129:626–38
Balkwill F, Charles KA, Mantovani A (2005) Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7:211–217
Lewis CE, Pollard JW (2006) Distinct role of macrophages in different tumor microenvironments. Cancer Res 66:605–612
Hagemann T, Robinson SC, Schulz M, Trumper L, Balkwill FR, Binder C (2004) Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis 8:1543–1549
Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, Heine H, Brandt E, Reiling N (2006) The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 108:965–973
Acknowledgement
We thank Annette Borchers and Florian Klemm for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pukrop, T., Binder, C. The complex pathways of Wnt 5a in cancer progression. J Mol Med 86, 259–266 (2008). https://doi.org/10.1007/s00109-007-0266-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-007-0266-2