Abstract
Background
Radiotherapy can induce cardiac injury in left-sided breast cancer cases. Cardiac-sparing irradiation using the deep inspiration breath-hold (DIBH) technique can achieve substantial dose reduction to vulnerable cardiac substructures compared with free breathing (FB). This study evaluated the dosimetric differences between both techniques at a single institution.
Methods
From 2017 to 2019, 130 patients with left-sided breast cancer underwent breast-conserving surgery (BCS; n = 121, 93.1%) or mastectomy (ME; n = 9, 6.9%) along with axillary lymph node staging (n = 105, 80.8%), followed by adjuvant irradiation in DIBH technique; adjuvant systemic therapy was included if applicable. 106 (81.5%) patients received conventional and 24 (18.5%) hypofractionated irradiation. Additionally, 12 patients received regional nodal irradiation. Computed tomography (CT) scans in FB and DIBH position were performed for all patients. Intrafractional 3D position monitoring of the patient surface in deep inspiration and breath gating was performed using Sentinel and Catalyst HD 3D surface scanning systems (C-RAD, Catalyst, C‑RAD AB, Uppsala, Sweden). Individual coaching and determination of breathing amplitude during the radiation planning CT was performed. Three-dimensional treatment planning was performed using standard tangential treatment portals (6 or 18 MV). The delineation of cardiac structures and both lungs was done in both the FB and the DIBH scan.
Results
All dosimetric parameters for cardiac structures were significantly reduced (p < 0.01 for all). The mean heart dose (Dmean) in the DIBH group was 1.3 Gy (range 0.5–3.6) vs. 2.2 Gy (range 0.9–8.8) in the FB group (p < 0.001). The Dmean for the left ventricle (LV) in DIBH was 1.5 Gy (range 0.6–4.5), as compared to 2.8 Gy (1.1–9.5) with FB (p < 0.001). The parameters for LV (V10 Gy, V15 Gy, V20 Gy, V23 Gy, V25 Gy, V30 Gy) were reduced by about 100% (p < 0.001). The LAD Dmean in the DIBH group was 4.1 Gy (range 1.2–33.3) and 14.3 Gy (range 2.4–37.5) in the FB group (p < 0.001). The median values for LAD such as V15 Gy, V20 Gy, V25 Gy, V30 Gy, and V40 Gy decreased by roughly 100% (p < 0.001). An increasing volume of left lung in the DIBH position resulted in dose sparing of cardiac structures.
Conclusion
For all ascertained dosimetric parameters, a significant dose reduction could be achieved in DIBH technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Incidental irradiation of the heart for left-sided breast cancer increases the rate of subsequent ischemic cardiac events [1]. It has been suggested that the mean heart dose correlates linearly with a relative increase in cardiac events of 7.4% without a threshold [2]. Radiation-induced cardiac impairment results from damage to the micro- and macrovasculature [3, 4]. Dose-dependent vulnerability of the entire left ventricle and all coronary segments justifies rigorous dose reduction [5,6,7,8]. This dose-dependent rise occurs after a few years and persists for at least two decades [2]. Notably, preexisting cardiac risk factors increase the absolute risk caused by radiation therapy (RT) [2].
The aggregated cardiac toxicity after multimodality therapy consisting of chemotherapy and RT has not been well studied [9]. Higher doses of anthracyclines combined with higher dose volumes of cardiac irradiation are associated with an increased risk of cardiac events [10]. However, in selected non-high-risk cardiac patients, the multimodal approach appears relatively safe [11].
Deep inspiration breath-hold technique (DIBH) in the supine position is a commonly used heart-sparing approach for radiotherapy [12]. DIBH can be performed by tangential 3D conformal radiotherapy (3DRT) or rotational/multiangle intensity-modulated radiotherapy (IMRT/VMAT) [13]. Alternatively, in selected patients with a low-risk profile, partial breast irradiation can be performed using external beam RT [14, 15], brachytherapy [16,17,18], or intraoperative radiation therapy (IORT) alone [19, 20]. DIBH-based RT allows a reproducible cardiac shift from the irradiation field, resulting in substantial dose reduction to cardiac structures [12, 13].
In the absence of published data from randomized trials of DIBH vs. free breathing (FB) RT in the supine position, reporting institutional experiences is necessary. The goal of this single-institutional retrospective study was to compare dosimetric outcomes between DIBH and FB for left-sided breast cancer patients.
Materials and methods
Patient selection and treatment planning
From December 2017 to December 2019, 203 patients with left-sided or bilateral breast cancer were screened for irradiation in DIBH technique and 130 patients were included in this analysis (Fig. 1). Ten participants were not able to comply with the requirements of the DIBH technique, the other 193 patients received CT scans in FB and DIBH (Fig. 1). After the DIBH vs. FB plan comparison before starting radiotherapy, 18 patients did not show any dosimetric benefit for cardiac structures, so FB RT was performed for them. No reasons could be identified beforehand; however, the most common reasons turned out to be thoracic anatomy, respiratory depth, or patient compliance. Another 36 DIBH RT patients were excluded from evaluation due to technical difficulties related to retroactively contouring cardiac structures. As such, for the purposes of this study, contouring and evaluation of the plans was only possible for 130 patients.
Institutional criteria for patient selection for a tumor bed boost included patients with breast-conserving surgery (BCS), premenopausal status, or postmenopausal status in addition to the following risk factors: tumor size ≥ 2 cm, extensive intraductal component, grade 3 disease, HER2-positive disease, and triple-negative breast cancer (TNBC).
BCS or mastectomy with sentinel lymph node excision or axillary nodal dissection was performed according to institutional protocols. Neoadjuvant or adjuvant chemotherapy as well as endocrine therapy was administered based on the currently accepted guidelines and individual recommendations of the interdisciplinary oncological board.
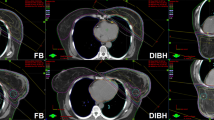
All patients were coached on the DIBH technique in the CT room using a surface image-guided RT (SGRT) system (C-RAD, Catalyst, C‑RAD AB, Uppsala, Sweden). The patients were asked to take a deep breath and hold it for a duration of 20 s, and the width of the gating window was set to 5 mm. The patients received CT scans (Brilliance, CT Big Bore, Philips, Cleveland, OH) in FB and DIBH with a slice thickness of 2 mm. CT-based three-dimensional treatment planning (Oncentra MasterPlan, Nucletron, Veenendaal, the Netherlands, and/or Eclipse™ planning systems, Varian Medical Systems, Palo Alto, CA, USA) was performed using standard tangential treatment portals (6 or 18 MV; Synergy; Elekta, Crawley, United Kingdom; Fig. 2).
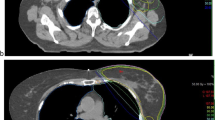
Isodose distribution in FB and DIBH plans for the same patient. a Axial scan in FB; b oblique reconstruction with visualization of tangential irradiation fields including the adaptations of the multileaf collimators “beam eye view,” 3DRT, 6 MV in FB; c axial scan in DIBH; d oblique reconstruction with visualization of tangential irradiation fields including the adapted of the multileaf collimators “beam eye view,” 3DRT, 6 MV in DIBH. 3DRT 3D conformal radiotherapy, DIBH deep inspiration breath-hold, FB free breathing
Subsequently, after completion of wound healing, adjuvant whole-breast irradiation (WBI) or thoracic wall RT was delivered according to standardized institutional protocols, which included hypofractionation (40.05 Gy in 15 fractions), conventional fractionation (50.0–50.4 Gy in 25–28 fractions), or with simultaneous integrated boost (58.8–61.6 Gy in 25–28 fractions). For indicated irradiation of lymph nodes (supraclavicular, axillary, internal mammary [IM]), a fractional dose of 1.7–1.8 Gy was used.
The institutional cardiac constraint was based on the following constraint of the DEGRO breast cancer expert panel: mean cardiac dose < 2.5 Gy [13]. An individualized decision was always made between heart doses and optimal target volume coverage, especially in the case of IM lymph node irradiation.
Volume delineation and dosimetric comparison
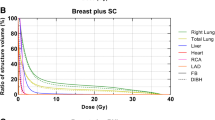
For the planned dosimetric evaluation, the following cardiac structures of interest were retrospectively delineated: left ventricle (LV), left anterior descending artery (LAD), and right coronary artery (RCA). The delineation was done in both the FB and the DIBH scan according to the RTOG recommendations and the atlas by Feng et al. [21]. DVH parameters were then assigned for left and right lung (volume, Dmean, D50%, Dmax, V5 Gy, V20 Gy, D20%, D30%), left ventricle (volume, Dmean, D50%, Dmax, D2%, V5 Gy, V10 Gy, V15 Gy, V20 Gy, V23 Gy, V25 Gy, V30 Gy, V40 Gy), LAD and RCA (Dmean, D50%, Dmax, D2%, V5 Gy, V10 Gy, V15 Gy, V20 Gy, V25 Gy, V30 Gy, V40 Gy), and heart (volume, Dmean, D50%, Dmax, D2%, V5 Gy, V10 Gy, V15 Gy, V20 Gy, V25 Gy, V30 Gy, V40 Gy).
Statistical analysis
Data are reported as a mean, median (range), and frequencies. For all dosimetric parameters, median values and their corresponding ranges as well as the relative dose reduction were determined. DVH parameters of the FB vs. DIBH plans were compared using either a paired t-test or a Wilcoxon signed-rank test. For evaluation of the impact of metric variables on dosimetric parameters (such as volumes of both lungs, heart, and cardiac subvolumes), univariate and multivariate analysis was performed using linear regression. Parameters that exhibited a p-value < 0.1 in univariate analysis were included in multivariate analysis.
P-values < 0.05 were considered statistically significant. Analysis was performed using SPSS version 27 (IBM, Armonk, NY, USA) and Microsoft Office Excel 2016 (Microsoft Corp. Redmond, WA, USA).
Results
Altogether, 130 patients with 260 CT scans were analyzed (Fig. 1). Table 1 displays the treatment characteristics of this population. Most patients had T1 disease (66.2%, n = 86) and were node negative (87.6%, n = 114). Twelve participants had simultaneous RT of the regional lymph nodes: ten received RT of the ipsilateral supraclavicular and axillary lymph nodes and two additional IM. The vast majority of patients underwent breast-conserving surgery (93.1%, n = 121). After adjuvant RT, no locoregional recurrences were observed at a median follow-up of 4 months.
Heart: LV, LAD, and RCA
The DVH parameters for heart structures and both lungs are summarized in Table 2 and Supplementary Table 1. All ascertained dosimetric parameters for all listed cardiac structures were significantly reduced (p < 0.01 for all) in the DIBH position (Table 2).
The mean heart dose (Dmean) in the DIBH group was 1.3 Gy (range 0.5–3.6) vs. 2.2 Gy (range 0.9–8.8) in the FB group. The mean heart Dmax dose in the DIBH group was reduced > 50% in comparison to the FB group. The mean values of V15 Gy, V20 Gy, V25 Gy, V30 Gy, and V40 Gy for heart in the DIBH cohort could be decreased by approximately 100% in comparison to the FB group.
The Dmean for LV in the DIBH technique was 1.5 Gy (range 0.6–4.5) and 2.8 Gy (1.1–9.5) with FB. In half of the patients, the LV Dmean was reduced by about 50% (Fig. 3).
The listed mean dosimetric values specifically for LV (V10 Gy, V15 Gy, V20 Gy, V23 Gy, V25 Gy, V30 Gy, V40 Gy) were reduced by approximately 100%.
The LAD Dmean in the DIBH group was on average 4.1 Gy (range 1.2–33.3) and 14.3 Gy (range 2.4–37.5) in the FB group. In half of the patients, the LAD Dmean was reduced by approximately 50% (Fig. 4). Consistently, the median values for LAD such as V15 Gy, V20 Gy, V23 Gy, V25 Gy, V30 Gy, and V40 Gy decreased by almost 100%.
The same trend was observed when considering the RCA Dmean: 1.0 Gy (range 0.4–1.9) in the DIBH group vs. 1.2 Gy (range 0.5–2.5) in FB group.
Lungs
With DIBH, the Dmean parameters of the left and right lungs were reduced by approximately 7% and 12%, respectively. The V5 Gy for the left lung in the DIBH group was 24.0% (range 11.2–44.3) vs. 24.9% (range 10.4–53.0) in the FB group. More pronounced was the observed V20 Gy reduction for the left lung with DIBH; on average 10.4% (range 0–22.9) vs. 12.1% (range 1.9–31.6). The right lung Dmean in the DIBH group was reduced by approximately 12% in comparison to the FB group.
The results of univariate and multivariate analyses are reported in Table 3. The left lung volume in the DIBH position was the independent variable in multivariate analysis (Table 3). When considering relevant dosimetric parameters based on the DEGRO breast cancer expert panel recommendations [13] such as heart Dmean, LV Dmean, LV V5 Gy, LAD Dmean, and LAD V30 Gy, only the left lung volume in the DIBH technique remained an independent predictor in multivariate analysis. Increasing left lung volumes (in DIBH) showed a dose-sparing effect (Table 3) only for LV V23 Gy and LAD V40 Gy and in the univariate analysis.
Discussion
Various studies have shown a benefit of DIBH in tangential 3DRT technique in regards to significant dose reduction of cardiac structures [22,23,24,25]. In line with previous studies, this single-institutional retrospective comparison demonstrates that the DIBH technique achieved a significant dose reduction for most analyzed dosimetric parameters with acceptable patient compliance.
All analyzed DVH parameters for all cardiac structures could be significantly reduced (p < 0.01 for all) in the DIBH position (Table 2). The heart Dmean in the DIBH group could be reduced by about > 40% in comparison to the FB group. Comparable with our results, Simonetto et al. showed reduction of mean heart doses by 35% (interquartile range 23–46%) in DIBH in comparison to FB [26]. The mean heart parameters for V15–V40 Gy in the DIBH cohort could be decreased by almost 100% in comparison to the FB group. Additionally, the LV Dmean with DIBH was 1.5 Gy (range 0.6–4.5) in contrast to 2.8 Gy (1.1–9.5) with FB. In half of the patients, LV Dmean was reduced by about 50% (Fig. 3).
The LAD Dmean in the DIBH group was 4.1 Gy (range 1.2–33.3) and 14.3 Gy (range 2.4–37.5) in the FB group. In contrast, Joo et al. demonstrated significantly superior Dmean reduction to the LAD, from 4079.1 cGy in FB to 2368.9 cGy in DIBH (p < 0.001), in comparison with our results [27]. Another study showed the mean LAD dose was 1.5 Gy with DIBH vs. 19.8 Gy with FB (p < 0.001), which may be more comparable with our values [28]. In half of the patients in our study, Dmean LAD was reduced by approximately 50% (Fig. 4).
Of note, the cardiopulmonary dose sparing for V50 Gy using the relative seriality model diminishes the likelihood of pneumonitis and cardiac mortality [29]. Our study demonstrates dose sparing in DIBH position by 100% for V40 Gy in heart (Table 2). Herein, long-term follow-up clinical data were not assessed. In breast cancer survivors, a radiation therapy-related mortality risk may persist for two decades and possibly even increase in the third [30]. In light of this, recent models show that in the presence of radiation-induced cardiac mortality, the mean expected years of life lost appears to be lower at 0.07 in DIBH cohorts vs. 0.11 in FB [26]. This DIBH effect was even more prominent in patients with high mean cardiac doses in FB (0.09 years for doses > 3 Gy vs. 0.02 years for doses < 1.5 Gy) [26]. Additionally, preexisting cardiovascular risk factors at baseline such as diabetes and smoking had a substantial impact on the 10-year cumulative risk for cardiovascular disease, which was not completely diminished using DIBH [31]. Unexpectedly, Jimenez et al. found that using modern adjuvant RT in ≥ 60-year-old women with right- or left-sided hormone receptor-positive early breast cancer is not associated with an increased risk of cardiac mortality within 10 years of RT [32]. Unfortunately, the authors did not provide any information on DIBH use [32].
DIBH decreased most DVH values for the left lung, in particular the Dmean by 7.3% and V20 Gy by 14.0% (Supplementary Table 1). Our results are similar to the study by Oechsner et al., wherein DIBH reduced the left lung Dmean by −19 ± 9% and the relative V20 Gy by −24 ± 10% [33]. Additionally, in our study, the left lung volume in DIBH was an independent predictor of reduced cardiac DVH parameters in multivariate analysis (Table 3), which is consistent with the results of the aforementioned study [33].
Accelerated partial breast irradiation is a possible alternative to whole-breast irradiation for selected women with a low-risk profile, as it can shorten treatment time and reduce radiation exposure to surrounding tissue [14, 15, 34]. A relevant prospective study showed that interstitial multicatheter APBI achieves equivalent local control compared to whole-breast RT [16]. The use of interstitial multicatheters may reduce the risk of late skin side effects [35]. However, when considering relevant dosimetric outcomes for LAD and left lung, the mean values are comparable to DIBH [36, 37]. However, the choice of APBI technique must be tailored to the location of tumor, treatment goals, and patient preferences [38].
Limitations of our study are, among others, the heterogeneity of delivered doses and treatment planning techniques and the lack of adjustment for existing cardiac risk factors. No adjustment was made for different dose/fractionation schemes. As the left lung volume was the only predictor of cardiac dose reduction, individual differences in respiratory fitness may be a major factor. There was no adjustment for this in our study. Optimal coaching of patients for DIBH may improve results further. We set the gating window to 5 mm to ensure robust radiation treatment and patient compliance and to reduce treatment time. However, with a smaller gating window, cardiac sparing might have been improved. Except for patients on trastuzumab, regular cardiologic follow-up was not conducted. Thus, the clinical outcome and relevance of cardiac dose reduction remains to be analyzed. Additionally, the screening of baseline cardiac risk factors and risk-adapted cardiological and radiotherapeutic follow-up should be adapted for the vulnerable risk groups. About 10% of patients derived little or no benefit from DIBH in terms of Dmean LAD. This may be related to suboptimal compliance with DIBH or individual anatomic circumstances, which is why we performed CT scans in DIBH and free breathing for all patients. The data can only be used for DIBH-radiation in breast cancer without elective lymph node irradiation, as the latter group is not adequately represented in this retrospective analysis.
Abbreviations
- ALND:
-
Axillary lymph node dissection
- APBI:
-
Accelerated partial breast irradiation
- BCS:
-
Breast conservation surgery
- CI:
-
Confidence interval
- DIBH:
-
Deep inspiration breath-hold
- 3DRT:
-
3D-Conformal radiotherapy
- DVH:
-
Dose–volume histogram
- ER:
-
Estrogen receptor
- FB:
-
Free breathing
- HER2:
-
Human epidermal growth factor receptor 2
- HT:
-
Hormonal therapy
- IM:
-
Internal mammary
- IMRT:
-
Intensity-modulated radiotherapy
- IORT:
-
Intraoperative radiotherapy
- LAD:
-
Left anterior descending artery
- LV:
-
Left ventricle
- MV:
-
Megavolt
- OS:
-
Overall survival
- RCA:
-
Right coronary artery
- RT:
-
Radiotherapy
- SEB:
-
Sequential boost
- SGRT:
-
Surface image-guided radiotherapy
- SIB:
-
Simultaneous integrated boost
- SLND:
-
Sentinel lymph node dissection
- TNBC:
-
Triple-negative breast cancer
- VMAT:
-
Volumetric modulated arc therapy
- WBI:
-
Whole-breast irradiation
References
Harris EE, Correa C, Hwang WT, Liao J, Litt HI, Ferrari VA et al (2006) Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol 24(25):4100–4106
Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D et al (2013) Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 368(11):987–998
Corn BW, Trock BJ, Goodman RL (1990) Irradiation-related ischemic heart disease. J Clin Oncol 8(4):741–750
Correa CR, Litt HI, Hwang WT, Ferrari VA, Solin LJ, Harris EE (2007) Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol 25(21):3031–3037
Taylor C, McGale P, Bronnum D, Correa C, Cutter D, Duane FK et al (2018) Cardiac structure injury after radiotherapy for breast cancer: cross-sectional study with individual patient data. J Clin Oncol 36(22):2288–2296
Wennstig AK, Garmo H, Isacsson U, Gagliardi G, Rintela N, Lagerqvist B et al (2019) The relationship between radiation doses to coronary arteries and location of coronary stenosis requiring intervention in breast cancer survivors. Radiat Oncol 14(1):40
Nilsson G, Holmberg L, Garmo H, Duvernoy O, Sjogren I, Lagerqvist B et al (2012) Distribution of coronary artery stenosis after radiation for breast cancer. J Clin Oncol 30(4):380–386
Skytta T, Tuohinen S, Boman E, Virtanen V, Raatikainen P, Kellokumpu-Lehtinen PL (2015) Troponin T‑release associates with cardiac radiation doses during adjuvant left-sided breast cancer radiotherapy. Radiat Oncol 10:141
van Dalen EC, van der Pal HJ, Kremer LC (2016) Different dosage schedules for reducing cardiotoxicity in people with cancer receiving anthracycline chemotherapy. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD005008.pub4
Shapiro CL, Hardenbergh PH, Gelman R, Blanks D, Hauptman P, Recht A et al (1998) Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients. J Clin Oncol 16(11):3493–3501
Magne N, Castadot P, Chargari C, Di Leo A, Philippson C, Van Houtte P (2009) Special focus on cardiac toxicity of different sequences of adjuvant doxorubicin/docetaxel/CMF regimens combined with radiotherapy in breast cancer patients. Radiother Oncol 90(1):116–121
Duma MN, Baumann R, Budach W, Dunst J, Feyer P, Fietkau R et al (2019) Heart-sparing radiotherapy techniques in breast cancer patients: a recommendation of the breast cancer expert panel of the German society of radiation oncology (DEGRO). Strahlenther Onkol 195(10):861–871
Piroth MD, Baumann R, Budach W, Dunst J, Feyer P, Fietkau R et al (2019) Heart toxicity from breast cancer radiotherapy : current findings, assessment, and prevention. Strahlenther Onkol 195(1):1–12
Meattini I, Marrazzo L, Saieva C, Desideri I, Scotti V, Simontacchi G et al (2020) Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast cancer: long-term results of the randomized phase III APBI-IMRT-florence trial. J Clin Oncol 38(35):4175–4183
Coles CE, Griffin CL, Kirby AM, Titley J, Agrawal RK, Alhasso A et al (2017) Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5‑year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 390(10099):1048–1060
Strnad V, Ott OJ, Hildebrandt G, Kauer-Dorner D, Knauerhase H, Major T et al (2016) 5‑year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet 387(10015):229–238
Lettmaier S, Kreppner S, Lotter M, Walser M, Ott OJ, Fietkau R et al (2011) Radiation exposure of the heart, lung and skin by radiation therapy for breast cancer: a dosimetric comparison between partial breast irradiation using multicatheter brachytherapy and whole breast teletherapy. Radiother Oncol 100(2):189–194
Novotna V, Sirak I, Pohankova D, Jandik P, Kasaova L, Grepl J et al (2021) Cardiac doses of accelerated partial breast irradiation with perioperative multicatheter interstitial brachytherapy. Strahlenther Onkol 197(4):288–295
Vaidya JS, Baum M, Tobias JS, D’Souza DP, Naidu SV, Morgan S et al (2001) Targeted intra-operative radiotherapy (Targit): an innovative method of treatment for early breast cancer. Ann Oncol 12(8):1075–1080
Vaidya JS, Baum M, Tobias JS, Massarut S, Wenz F, Murphy O et al (2006) Targeted intraoperative radiotherapy (TARGIT) yields very low recurrence rates when given as a boost. Int J Radiat Oncol Biol Phys 66(5):1335–1338
Feng M, Moran JM, Koelling T, Chughtai A, Chan JL, Freedman L et al (2011) Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys 79(1):10–18
Pedersen AN, Korreman S, Nystrom H, Specht L (2004) Breathing adapted radiotherapy of breast cancer: reduction of cardiac and pulmonary doses using voluntary inspiration breath-hold. Radiother Oncol 72(1):53–60
Schonecker S, Walter F, Freislederer P, Marisch C, Scheithauer H, Harbeck N et al (2016) Treatment planning and evaluation of gated radiotherapy in left-sided breast cancer patients using the Catalyst(TM)/Sentinel(TM) system for deep inspiration breath-hold (DIBH). Radiat Oncol 11(1):143
Vikstrom J, Hjelstuen MH, Wasbo E, Mjaaland I, Dybvik KI (2018) A comparison of conventional and dynamic radiotherapy planning techniques for early-stage breast cancer utilizing deep inspiration breath-hold. Acta Oncol 57(10):1325–1330
Chatzikonstantinou G, Scherf C, Kohn J, Ackermann H, Ramm U, Tselis N (2021) Matched-pair dosimetric comparison of cardiac radiation exposure between deep-inspiration breath-hold whole-breast radiation therapy with Active Breathing Coordinator and interstitial multicatheter high-dose-rate brachytherapy as accelerated partial breast irradiation in adjuvant treatment of left-sided breast cancer after breast-conserving surgery. Strahlenther Onkol 197(4):308–316
Simonetto C, Eidemuller M, Gaasch A, Pazos M, Schonecker S, Reitz D et al (2019) Does deep inspiration breath-hold prolong life? Individual risk estimates of ischaemic heart disease after breast cancer radiotherapy. Radiother Oncol 131:202–207
Joo JH, Kim SS, Ahn SD, Kwak J, Jeong C, Ahn SH et al (2015) Cardiac dose reduction during tangential breast irradiation using deep inspiration breath hold: a dose comparison study based on deformable image registration. Radiat Oncol 10:264
Sakyanun P, Saksornchai K, Nantavithya C, Chakkabat C, Shotelersuk K (2020) The effect of deep inspiration breath-hold technique on left anterior descending coronary artery and heart dose in left breast irradiation. Radiat Oncol J 38(3):181–188
Korreman SS, Pedersen AN, Aarup LR, Nottrup TJ, Specht L, Nystrom H (2006) Reduction of cardiac and pulmonary complication probabilities after breathing adapted radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys 65(5):1375–1380
Henson KE, McGale P, Taylor C, Darby SC (2013) Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer 108(1):179–182
Gaasch A, Schonecker S, Simonetto C, Eidemuller M, Pazos M, Reitz D et al (2020) Heart sparing radiotherapy in breast cancer: the importance of baseline cardiac risks. Radiat Oncol 15(1):117
Jimenez RB, Wong SM, Johnson A, Lalani N, Hughes KS (2021) The association between cardiac mortality and adjuvant radiation therapy among older patients with stage I estrogen positive breast cancer: a surveillance, epidemiology, and end results (SEER)-based study on cardiac mortality and radiation therapy. Adv Radiat Oncol 6(2):100633
Oechsner M, Dusberg M, Borm KJ, Combs SE, Wilkens JJ, Duma MN (2019) Deep inspiration breath-hold for left-sided breast irradiation: analysis of dose-mass histograms and the impact of lung expansion. Radiat Oncol 14(1):109
Stoian R, Erbes T, Zamboglou C, Scholber J, Gainey M, Sachpazidis I et al (2021) Intraoperative radiotherapy boost as part of breast-conservation therapy for breast cancer: a single-institution retrospective analysis. Strahlenther Onkol 197(9):812–819
Polgar C, Ott OJ, Hildebrandt G, Kauer-Dorner D, Knauerhase H, Major T et al (2017) Late side-effects and cosmetic results of accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: 5‑year results of a randomised, controlled, phase 3 trial. Lancet Oncol 18(2):259–268
Witt JS, Gao RW, Sudmeier LJ, Rosenberg SA, Francis DM, Wallace CR et al (2019) Low cardiac and left anterior descending coronary artery dose achieved with left-sided multicatheter interstitial-accelerated partial breast irradiation. Brachytherapy 18(1):50–56
Major T, Stelczer G, Pesznyak C, Meszaros N, Polgar C (2017) Multicatheter interstitial brachytherapy versus intensity modulated external beam therapy for accelerated partial breast irradiation: A comparative treatment planning study with respect to dosimetry of organs at risk. Radiother Oncol 122(1):17–23
Patel RR, Becker SJ, Das RK, Mackie TR (2007) A dosimetric comparison of accelerated partial breast irradiation techniques: multicatheter interstitial brachytherapy, three-dimensional conformal radiotherapy, and supine versus prone helical tomotherapy. Int J Radiat Oncol Biol Phys 68(3):935–942
Funding
The authors received no specific funding for this study.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
T.S., J.W., N.H.N.: study conception and study design. J.W., S.S., J.L., T.R., M.S., J.S., C.Z., E.G., D.B., I.J‑B., A‑L.G., N.N, T.S.: data acquisition, data analysis and data interpretation. J.W., J.L., T.R., M.S. performed DIBH coaching and treatment planning. J.W and T.S.: statistical analysis. D.B., V.V., D.K., A‑L.G., N.N., T.S.: manuscript editing. C.Z., E.G., D.B., V.V., D.K., A‑L.G., N.N., T.S.: manuscript reviewed.
Corresponding author
Ethics declarations
Conflict of interest
J. Wolf, S. Stoller, J. Lübke, T. Rothe, M. Serpa, J. Scholber, C. Zamboglou, E. Gkika, D. Baltas, I. Juhasz-Böss, V. Verma, A.-L. Grosu, and N.H. Nicolay declare that they have no competing interests. D. Krug, has received honoraria from Merck Sharp & Dohme outside the submitted work. T. Sprave has received honoraria from Hologic outside the submitted work.
Ethical standards
The study was approved by the institutional ethical review committee (reference no. 453/19). Consent for publication: not applicable.
Additional information
Availability of data and materials
The data used in this analysis are available with the authors’ permission.
Supplementary Information
66_2022_1998_MOESM1_ESM.docx
Supplement: Table 1: Comparison of selected DVH parameters of both lungs in DIBH and FB techniques. Comparison of absolute mean values (ranges) of DVH parameters for both lungs and relative changes in percent between DIBH and FB techniques using two-sided significances of changes in distributions of these measures
66_2022_1998_MOESM2_ESM.docx
Supplement: Table 2: Univariate and multivariate analysis for selected DVH parameters from heart structures in DIBH position. DIBH deep inspiration breath-hold; DVH dose–volume histogram; FB free breathing; LAD left anterior descending artery; LV left ventricle; RCA right coronary artery
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wolf, J., Stoller, S., Lübke, J. et al. Deep inspiration breath-hold radiation therapy in left-sided breast cancer patients: a single-institution retrospective dosimetric analysis of organs at risk doses. Strahlenther Onkol 199, 379–388 (2023). https://doi.org/10.1007/s00066-022-01998-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-022-01998-z