Abstract
Purpose
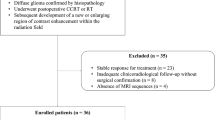
Noninvasive methods are desired to predict the treatment response to Stereotactic Radiosurgery (SRS) to improve individual tumor management. In a previous study, we demonstrated that Diffusion Tensor Imaging (DTI)-derived parameter maps significantly correlate to SRS response. This study aimed to analyze and compare the predictive value of intratumoral ADC and DTI parameters in patients with meningiomas undergoing radiosurgery.
Methods
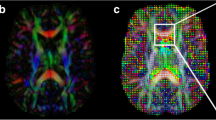
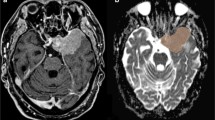
MR images of 70 patients treated with Gamma Knife SRS for WHO grade I meningiomas were retrospectively reviewed. MR acquisition included pre- and post-treatment DWI and DTI sequences, and subtractions were calculated to assess for radiation-induced changes in the parameter values.
Results
After a mean follow-up period (FUP) of 52.7 months, 69 of 70 meningiomas were controlled, with a mean volume reduction of 34.9%. Whereas fractional anisotropy (FA) values of the initial exam showed the highest correlation to tumor volume change at the last FU (CC = − 0.607), followed by the differences between first and second FU values of FA (CC = − 0.404) and the first longitudinal diffusivity (LD) value (CC = − 0.375), the correlation coefficients of all ADC values were comparably low. Nevertheless, all these correlations, except for ADC measured at the first follow-up, reached significance.
Conclusion
For the first time, the prognostic value of ADC maps measured in meningiomas before and at first follow-up after Gamma Knife SRS, was compared to simultaneously acquired DTI parameter maps. Quantities assessed from ADC maps present significant correlations to the volumetric meningioma response but are less effective than correlations with DTI parameters.


Similar content being viewed by others
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Rogers L, Barani I, Chamberlain M, Kaley TJ, McDermott M, Raizer J et al (2015) Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO rev J Neurosurg 122(1):4–23
Cohen-Inbar O, Lee CC, Sheehan JP (2016) The contemporary role of stereotactic radiosurgery in the treatment of meningiomas. Neurosurg Clin N Am 27(2):215–28
Ius T, Tel A, Minniti G, Somma T, Solari D, Longhi M et al (2021) Advances in multidisciplinary management of skull base meningiomas. Cancers (Basel) 13(11):2664
Sheehan J, Pikis S, Islim AI, Chen CJ, Bunevicius A, Peker S et al (2022) An international multicenter matched cohort analysis of incidental meningioma progression during active surveillance or after stereotactic radiosurgery: the IMPASSE study. Neuro Oncol 24(1):116–124
Fatima N, Meola A, Pollom E, Chaudhary N, Soltys S, Chang SD (2019) Stereotactic radiosurgery in large intracranial meningiomas: a systematic review. World Neurosurg 129:269–275
Helis CA, Hughes RT, Cramer CK, Tatter SB, Laxton AW, Bourland JD et al (2020) Stereotactic radiosurgery for atypical and anaplastic meningiomas. World Neurosurg 144:e53-61
DiBiase SJ, Kwok Y, Yovino S, Arena C, Naqvi S, Temple R et al (2004) Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys 60(5):1515–9
Starke RM, Nguyen JH, Rainey J, Williams BJ, Sherman JH, Savage J et al (2011) Gamma Knife surgery of meningiomas located in the posterior fossa: factors predictive of outcome and remission. J Neurosurg 114(5):1399–1409
Santacroce A, Walier M, Régis J, Liščák R, Motti E, Lindquist C et al (2012) Long-term tumor control of benign intracranial meningiomas after radiosurgery in a series of 4565 patients. Neurosurgery 70(1):32–39
Sheehan JP, Starke RM, Kano H, Kaufmann AM, Mathieu D, Zeiler FA et al (2014) Gamma knife radiosurgery for sellar and parasellar meningiomas: a multicenter study. J Neurosurg 120(6):1268–1277
Mansouri A, Larjani S, Klironomos G, Laperriere N, Cusimano M, Gentili F et al (2015) Predictors of response to gamma knife radiosurgery for intracranial meningiomas. J Neurosurg 123(5):1294–1300
Speckter H, Radulovic M, Trivodaliev K, Vranes V, Joaquin J, Hernandez W et al (2022) MRI radiomics in the prediction of the volumetric response in meningiomas after gamma knife radiosurgery. J Neurooncol 159(2):281–291
Filippi CG, Edgar MA, Uluğ AM, Prowda JC, Heier LA, Zimmerman RD (2001) Appearance of meningiomas on diffusion-weighted images: correlating diffusion constants with histopathologic findings. AJNR Am J Neuroradiol 22(1):65–72
Kashimura H, Inoue T, Ogasawara K, Arai H, Otawara Y, Kanbara Y et al (2007) Prediction of meningioma consistency using fractional anisotropy value measured by magnetic resonance imaging. J Neurosurg 107:784–787
Tropine A, Dellani PD, Glaser M, Bohl J, Plöner T, Vucurevic G et al (2007) Differentiation of fibroblastic meningiomas from other benign subtypes using diffusion tensor imaging. J Magn Reson Imaging 25(4):703–708
Yan PF, Yan L, Hu TT, Xiao DD, Zhang Z, Zhao HY et al (2017) The potential value of preoperative mri texture and shape analysis in grading meningiomas: a preliminary investigation. Transl Oncol 10(4):570–577
Coroller TP, Bi WL, Huynh E, Abedalthagafi M, Aizer AA, Greenwald NF et al (2017) Radiographic prediction of meningioma grade by semantic and radiomic features. PLoS ONE 12(11):e0187908
Hale AT, Stonko DP, Wang L, Strother MK, Chambless LB (2018) Machine learning analyses can differentiate meningioma grade by features on magnetic resonance imaging. Neurosurg Focus 45(5):E4
Kalasauskas D, Kronfeld A, Renovanz M, Kurz E, Leukel P, Krenzlin H et al (2020) Identification of high-risk atypical meningiomas according to semantic and radiomic features. Cancers (Basel) 12(10):2942
Speckter H, Bido J, Hernandez G, Mejía DR, Suazo L, Valenzuela S et al (2016) Prognostic value of diffusion tensor imaging parameters for Gamma Knife radiosurgery in meningiomas. J Neurosurg 125(Supplement_1):83–88
Berberat J, Roelcke U, Remonda L, Schwyzer L (2021) Long-term apparent diffusion coefficient value changes in patients undergoing radiosurgical treatment of meningiomas. Acta Neurochir (Wien) 163(1):89–95
Speckter H, Bido J, Hernandez G, Rivera D, Suazo L, Valenzuela S et al (2018) Pretreatment texture analysis of routine MR images and shape analysis of the diffusion tensor for prediction of volumetric response after radiosurgery for meningioma. J Neurosurg 129(Suppl1):31–37
Changizi V, Kadhum MJ, Taher HJ, Najim HS, Saroush HA (2021) Grading meningiomas by used imaging features on magnetic resonance imaging. Clin Schizophr Relat Psychoses. https://doi.org/10.3371/CSRP.CVKM.081221
Patibandla MR, Lee CC, Tata A, Addagada GC, Sheehan JP (2018) Stereotactic radiosurgery for WHO grade I posterior fossa meningiomas: long-term outcomes with volumetric evaluation. J Neurosurg 129(5):1249–1259
McMahon SJ (2018) The linear quadratic model: usage, interpretation and challenges. Phys Med Biol 64(1):01TR01
Speckter H, Santana J, Miches I, Hernandez G, Bido J, Rivera D et al (2019) Assessment of the alpha/beta ratio of the optic pathway to adjust hypofractionated stereotactic radiosurgery regimens for perioptic lesions. J Radiat Oncol 8(3):279–289
Vernimmen FJAI, Slabbert JP (2010) Assessment of the α/ß ratios for arteriovenous malformations, meningiomas, acoustic neuromas, and the optic chiasma. Int J Radiat Biol 86(6):486–498
Piper K, Yu S, Taghvaei M, Fernandez C, Mouchtouris N, Smit RD, Yudkoff C, Collopy S, Reyes M, Lavergne P, Karsy M, Prashant GN, Shi W, Evans J (2022) Radiation of meningioma dural tail may not improve tumor control rates. Front Surg 9:908745. https://doi.org/10.3389/fsurg.2022.908745. (PMID: 35860199; PMCID: PMC9289604)
Huang RY, Bi WL, Weller M, Kaley T, Blakeley J, Dunn I et al (2019) Proposed response assessment and endpoints for meningioma clinical trials: report from the response assessment in neuro-oncology working group. Neuro Oncol 21(1):26–36
Harrison G, Kano H, Lunsford D, Flickinger JC, Kondziolka D (2015) Quantitative tumor volume responses after Gamma Knife radiosurgery for meningiomas. J Neurosurg 124:146–154
Corporation IBM (2019) SPSS statistics for Windows. Armonk, NY
Surov A, Gottschling S, Mawrin C, Prell J, Spielmann RP, Wienke A et al (2015) Diffusion-weighted imaging in meningioma: prediction of tumor grade and association with histopathological parameters. Transl Oncol 8(6):517–523
Winston GP (2012) The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg 2(4):254–265
Camargo A, Schneider T, Liu L, Pakpoor J, Kleinberg L, Yousem DM (2017) Pretreatment ADC values predict response to radiosurgery in vestibular schwannomas. Am J Neuroradiol 38(6):1200–1205
Ko CC, Zhang Y, Chen JH, Chang KT, Chen TY, Lim SW et al (2021) Preoperative MRI radiomics for the prediction of progression and recurrence in meningiomas. Front Neurol 12:636235
Feraco P, Scartoni D, Porretti G, Pertile R, Donner D, Picori L et al (2021) Predict treatment response by magnetic resonance diffusion weighted imaging: a preliminary study on 46 meningiomas treated with proton-therapy. Diagnostics 11(9):1684
Franconeri A, Sacco S, Raciti MV, Maggi A, Muzic SI, Imparato S et al (2021) Intravoxel incoherent motion as a tool to detect early microstructural changes in meningiomas treated with proton therapy. Neuroradiology 63(7):1053–1060
Yin B, Liu L, Zhang BY, Li YX, Li Y, Geng DY (2012) Correlating apparent diffusion coefficients with histopathologic findings on meningiomas. Eur J Radiol 81(12):4050–4056
Cha S (2006) Update on brain tumor imaging: from anatomy to physiology. AJNR Am J Neuroradiol 27:475–487
Lin X, Lee M, Buck O, Woo KM, Zhang Z, Hatzoglou V et al (2017) Diagnostic accuracy of T1-weighted dynamic contrast-enhanced–MRI and DWI-ADC for differentiation of glioblastoma and primary CNS lymphoma. Am J Neuroradiol 38(3):485–491
Drake-Pérez M, Boto J, Fitsiori A, Lovblad K, Vargas MI (2018) Clinical applications of diffusion weighted imaging in neuroradiology. Insights Imaging 9(4):535–547
Schwyzer L, Berberat J, Remonda L, Roelcke U (2015) Susceptibility changes in meningiomas influence the apparent diffusion coefficient in diffusion-weighted MRI. J Neuroradiol 42(6):332–337
Surov A, Meyer HJ, Wienke A (2017) Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: a meta-analysis. Oncotarget 8(35):59492–59499
Campbell A, Davis LM, Wilkinson SK, Hesketh RL (2019) Emerging functional imaging biomarkers of tumour responses to radiotherapy. Cancers (Basel) 11(2):131
Eriksson D, Stigbrand T (2010) Radiation-induced cell death mechanisms. Tumor Biol 31(4):363–372
Barker HE, Paget JTE, Khan AA, Harrington KJ (2015) The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 15(7):409–425
Mahmood F, Johannesen HH, Geertsen P, Hansen RH (2017) Repeated diffusion MRI reveals earliest time point for stratification of radiotherapy response in brain metastases. Phys Med Biol 62(8):2990–3002
Hein PA, Eskey CJ, Dunn JF, Hug EB (2004) Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol 25:201–209
Funding
This study has not received any financial support.
Author information
Authors and Affiliations
Contributions
Conception and design: HS and PS, Data collection: All authors, Data analysis and interpretation: HS, SPS, RMG, PS, Manuscript writing: HS, SPS, RMG, PS, All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Speckter, H., Palque-Santos, S., Mota-Gonzalez, R. et al. Can Apparent Diffusion Coefficient (ADC) maps replace Diffusion Tensor Imaging (DTI) maps to predict the volumetric response of meningiomas to Gamma Knife Radiosurgery?. J Neurooncol 161, 547–554 (2023). https://doi.org/10.1007/s11060-023-04243-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04243-4