Abstract
Background
It is unknown whether technological advancement of stent-retriever devices influences typical observational indicators of safety or effectiveness.
Methods
Observational retrospective study of APERIO® (AP) vs. new generation APERIO® Hybrid (APH) (Acandis®, Pforzheim, Germany) stent-retriever device (01/2019–09/2020) for mechanical thrombectomy (MT) in large vessel occlusion (LVO) stroke. Primary effectiveness endpoint was successful recanalization eTICI (expanded Thrombolysis In Cerebral Ischemia) ≥ 2b67, primary safety endpoint was occurrence of hemorrhagic complications after MT. Secondary outcome measures were time from groin puncture to first pass and successful reperfusion, and the total number of passes needed to achieve the final recanalization result.
Results
A total of 298 patients with LVO stroke who were treated by MT matched the inclusion criteria: 148 patients (49.7%) treated with AP vs. 150 patients (50.3%) treated with new generation APH. Successful recanalization was not statistically different between both groups: 75.7% for AP vs. 79.3% for APH; p = 0.450. Postinterventional hemorrhagic complications and particularly subarachnoid hemorrhage as the entity possibly associated with stent-retriever device type was significantly less frequent in the group treated with the APH: 29.7% for AP and 16.0% for APH; p = 0.005; however, rates of symptomatic hemorrhage with clinical deterioration and in domo mortality were not statistically different. Neither the median number of stent-retriever passages needed to achieve final recanalization, time from groin puncture to first pass, time from groin puncture to final recanalization nor the number of cases in which successful recanalization could only be achieved by using a different stent-retriever as bail-out device differed between both groups.
Conclusion
In the specific example of the APERIO® stent-retriever device, we observed that further technological developments of the new generation device were not associated with disadvantages with respect to typical observational indicators of safety or effectiveness.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Mechanical thrombectomy (MT) has become the standard of care for large vessel occlusion (LVO) in acute ischemic stroke [1,2,3]. In 2015 there were 5 large randomized trials (MR CLEAN, ESCAPE, REVASCAT, SWIFT PRIME and EXTEND IA) that first proved the efficacy of MT, each trial mainly employing stent-retriever devices to achieve recanalization [4,5,6,7,8]. Hence, current consensus statements and/or guidelines advocate the use of stent-retriever devices, which continues to be the predominant endovascular technical approach to achieve successful recanalization in LVO stroke [1, 2]. The most widely practiced techniques utilize stent-retriever embolectomy as the central procedural step of MT supported by one of several possible techniques of proximal occlusion/aspiration and/or distal aspiration. Subsequently, first randomized controlled trials tested distal aspiration alone, not with the aim to test whether it was superior to completely dispense with the use of a stent-retriever device but for raising evidence that this alternative probably is non-inferior [9, 10]. To date, a clear superiority of any single technique or device does not seem to emerge. There is a wide selection of different stent retriever devices available from different manufacturers. Over the last few years medical progress of endovascular stroke treatment has been paralleled by dynamic innovations in stent-retriever device technology. This led to both novel devices and also further technical refinements of first generation devices, such as modifications in material composition and their structural design. These advancements in medical device technology and engineering also represent potential improvements for interventional handling and also seem promising to have positive impact on recanalization effectiveness and safety; however, these assumptions need to be tested by studies with observational design and/or registries. Our retrospective analysis addressed this question by investigating whether stent-retriever device evolution would be associated with alterations in typical observational endpoints of safety or effectiveness in the specific example of two consecutive generations of one particular stent-retriever device (APERIO®, Acandis®, Pforzheim, Germany).
Methods
Patient Selection
We retrospectively included all consecutive patients with acute symptomatic LVO stroke who were treated by MT at our comprehensive stroke center (University Hospital Würzburg, Germany) between January 2019 and September 2020, using either the APERIO® (AP) or the APERIO® Hybrid (APH) stent-retriever device. Our institution serves as a center for teleneurological and teleradiological stroke medicine, including diagnosis, assessment of eligibility for MT, coordination of referral and subsequent MT with 24 h/7 day access to the angiography suite in a regional network in north-western Bavaria [11]. Informed consent was waived with approval by the local ethics committee. Occlusions of both the anterior (middle cerebral artery, MCA, anterior cerebral artery, ACA, distal internal carotid artery, ICA) and posterior (basilar artery, BA, vertebral artery, VA) circulations in the setting of acute ischemic stroke were taken into account.
Clinical and Radiological Assessment
Clinical stroke severity as assessed by the National Institutes of Health Stroke Scale (NIHSS [12]) was evaluated by trained neurologists before (baseline) and after endovascular treatment (follow-up after 24, 48 and 72 h). All patients underwent a non-contrast computed tomography (CT) scan, CT angiography (CTA) and, if indicated, additional CT perfusion prior to endovascular treatment. Radiological evaluation of non-contrast CT scans was done by trained neuroradiologists using the Alberta Stroke Program Early Computed Tomography Score (ASPECTS) before and after MT for LVO in the anterior circulation [13]. Collateral status was evaluated for CT and digital subtraction angiography (DSA) imaging using both the score of Miteff et al. for CTA [14] and the score of the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) for DSA [15]. Endovascular treatment was evaluated using the expanded thrombolysis in cerebral infarction (eTICI) score [16], the total number of stent-retriever passes needed as well as the time intervals from groin to first pass and to final eTICI (in minutes). Primary safety endpoints were hemorrhagic complications after MT. To account for substantial heterogeneity in how symptomatic intracranial hemorrhage (sICH) is defined in the literature, we proceeded in the following manner: for all patients we applied the morphological classification of bleeding events according to the Heidelberg bleeding classification (HBC) [17], which is broadly consistent with the commonly used European cooperative acute stroke study (ECASS) II classification of hemorrhagic complications [18]. Ratings were performed by board-certified neuroradiologists and advanced neuroradiology fellows (AM, MS). We finally applied different definitions of sICH: first, we used the ECASS II definition of concomitant occurrence of any extravascular blood in the brain or within the cranium and clinical worsening (≥ 4 points on the NIHSS) [19], which seems to overestimate bleeding complications. Secondly, we defined sICH in accordance with the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST [20]) as concomitant occurrence of clinical worsening (≥ 4 points on the NIHSS) and evidence of hemorrhage rated PH2 (blood clots in more than 30% of the infarcted area with substantial space-occupying effect, according to ECASS), which is congruent with class 2, according to HBC. In order to differentiate reperfusion-related hemorrhage or hemorrhage into pre-existing parenchymal damage from potentially device attributable hemorrhage, we hypothesized that local subarachnoid hemorrhage (SAH) could possibly be associated with mechanical impact and thus be influenced by the specific device. It was our primary intention to investigate such possible influences by observation. As a novelty and with the aim to address this specific question, we defined possibly device-attributable symptomatic hemorrhage as the simultaneous occurrence of clinical worsening and CT-graphically detectable local subarachnoid hemorrhage (=symptomatic subarachnoid hemorrhage, sSAH) after MT. According to Liebeskind et al. successful reperfusion was defined as eTICI ≥ 2b67 in final angiographic control [21].
Device Selection
At our institution the new generation APH thrombectomy device was introduced for MT in 09/2019, thus replacing its first-generation predecessor (AP). During the period of observation, the first-generation AP device was employed during 01/2019 to 04/2020. There was an overlapping interval of 6 months in which both stent-retriever devices were available and where the decision to use one of these two devices was at the discretion of the neurointerventionalist.
APERIO® and the New Generation APERIO® Hybrid Stent-retriever Device
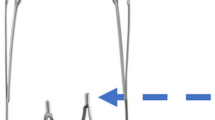
Both devices feature a hybrid cell design with smaller closed cells for better wall apposition and expansion into the clot and larger open cells that enable integration into the thrombus. Integrated anchoring elements provide additional support for atraumatic clot retention (Fig. 1).
The new generation APH but not the first-generation AP features full-length visibility while maintaining its hybrid cell design. This was realized by braiding two radiopaque wires into the stent structure. These two wires are composed of nitinol and a platinum core to ensure elasticity and, at the same time, to optimize fluoroscopic visibility. Unlike the AP, the APH thrombectomy device works without a transport wire and without distal wire tip, which reaches through and beyond the stent-retriever in the first-generation AP device. The radiopaque marker concept of the APH is supplemented with one proximal and three distal radiopaque markers, which consist of platinum iridium for improved visualization in comparison to the gold markers of the AP (Fig. 2).
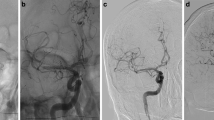
Fluoroscopic visibility of the deployed APERIO® stent retriever device (a) and the APERIO® Hybrid stent-retriever device ex vivo (b), provided upon request by courtesy of Acandis®, and the APERIO® Hybrid stent-retriever device deployed (c). Please note that unlike the AP, the APH is deployed without a distal wire tip and features substantially improved visibility over its full length with one proximal and three distal radiopaque markers
In order to evaluate the intraprocedural handling of both devices including aspects of visibility and navigability, a standardized questionnaire was answered by four participating neurointerventionalists. Visibility and tractability were retrospectively rated on a 4-point Likert scale (poor—neutral—good—very good).
The new generation APH comes in two additional sizes: 4.5 × 50 mm and 6.0 × 50 mm and has a longer transport wire of 212 cm compared to the AP (200 cm). Also, the radial force of the new generation APH is higher than the radial force of AP over all device sizes except the upper range of 4.5 × 30 mm.
Procedure
All MT procedures were performed by board-certified neurointerventional experts or by supervised neurointerventional fellows in a biplane neuroangiography suite (Axiom Artis Q, Siemens Healthcare, Erlangen, Germany). All patients were under general anesthesia when treated. Endovascular access was obtained via transfemoral approach through the common femoral artery (CFA) using the Seldinger technique. In two cases transfemoral access to the target LVO lesion was not possible and required a transbrachial (n = 1) or transcervical (n = 1) access route. Nontherapeutic doses of 1000 IU unfractionated heparin were added to 1000 ml rinsing fluid (standard 0.9% saline solution) to avoid catheter-associated thrombus formation. A flexible aspiration catheter (e.g. SOFIA 5F or 6F, 125 cm length, MicroVention, Tustin, CA, USA) was biaxially advanced into the cervical segment of the internal carotid artery or the vertebral artery via guide catheter (e.g. Vista Brite tip, Cordis, Miami, FL, USA or IVA6F80, Balt, Montmorency, France). After visualization of the thromboembolic occlusion, a microcatheter (e.g. 21 or 27 Neuroslider®, Acandis) was advanced through the occluding thrombus over a microguidewire (e.g. Traxcess, MicroVention), a stent-retriever device was deployed and the aspiration catheter was placed in close proximal vicinity to the thrombus/embolus.
As per institutional standard, we applied stent-retriever deployment and stent-embolus retrieval supported by aspiration via an intermediate catheter advanced to the proximal end of the stent-retriever (supported also by manual aspiration at the proximal guiding catheter) [22, 23].
Statistical Analysis
We used standard descriptive statistical measures including mean ± standard deviation (SD) or median with interquartile range (IQR) for numerical data and absolute or relative frequency distribution for categorical variables. Depending on the parametric or nonparametric distribution of data, intergroup comparison was performed using Student’s t‑test or Mann-Whitney U‑test for continuous variables, χ2-test or Fisher’s exact test for categorical variables. A two-sided p-value < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics for Windows (Version 26.0, IBM, Armonk, NY, USA).
Results
Baseline Characteristics
A total of 298 patients matched the inclusion criteria. The median age was 78 years (IQR 66–83 years), 54.0% were female and median baseline NIHSS was 14 (IQR 9–17). Of the patients 148 (56.1% female; median age: 78 years) underwent MT using the AP thrombectomy device vs. 150 patients (52.0% female; median age: 78 years) using the APH thrombectomy device. There were no significant differences in the following baseline variables: sex, age, NIHSS, cardiovascular risk factors (arterial hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, positive family history, smoking) and baseline anticoagulation or antiplatelet therapy (Table 1). A total of 64 (43.2%) patients of the AP group vs. 55 (36.7%) patients of the APH group received intravenous alteplase prior to endovascular treatment (p = 0.246). The distribution of vessel occlusion sites differed slightly but statistically significantly as LVO in the anterior circulation was more frequent in the AP group (95.3% vs. 86.7% in the APH group; p = 0.009). Occlusion of the basilar artery was more frequent in the APH group (AP: 3.4% vs. APH: 10.7%; p = 0.014).
Radiological/Angiographic Characteristics
Patients treated with the new generation APH had a statistically significantly lower ASPECT score in the CT scan prior to MT (p = 0.010) with nominal median ASPECT scores of 8 (IQR 7–9) for both groups. Collateral scores in both CT and conventional angiography were not significantly different in both groups. Stenting of ICA tandem stenosis at cervical level was necessary in 19 (12.8%) patients of the AP group compared to 6 (4.0%) patients of the APH group (p = 0.006). Intracranial bail-out stenting to preserve patency of underlying intracranial stenosis was performed in one single patient treated with the new generation APH thrombectomy device. Tractability and visibility of the AP and APH were retrospectively rated by 4 of our interventionalists. With regards to tractability, both the AP and the new APH were equally rated as “good” by one and “very good” by 3 of our 4 interventionalists. Visibility of the AP, on the other hand, was rated as “neutral” or “good” by 2 interventionalists each, while visibility of the new APH was rated as “very good” by all 4 participating neurointerventionalists.
Effectiveness
Successful recanalization (eTICI ≥ 2b67) was achieved in 112 (75.7%) patients of the AP group and in 119 (79.3%) patients of the APH group, revealing no significant differences in recanalization rates between the two devices (p = 0.450). A median of 2 passages (IQR 1–3) were needed to achieve successful recanalization in both groups. In 8/150 (5.3%) patients treated with the new generation APH recanalization was not successful despite using different bail-out thrombectomy devices (vs. 2/148, 1.4%, patients in the AP group, p = 0.056). In 4/148 (2.7%) patients treated with the AP and in 6/150 (4.0%) patients treated with the new generation APH device secondary distal occlusions occurred within the same territory after thrombectomy of the primary target LVO lesion and were treated with secondary distal thrombectomy. In these cases, distal recanalization was possible using a smaller thrombectomy device (Catch Mini 3 × 15 mm, Balt, Montmorency, France) in 2/4 patients of the AP group and in 6/6 patients of the APH group. In 5/150 (3.3%) patients treated with the new generation APH device successful recanalization was achieved only after switching to another thrombectomy device vs. 3 (2.0%) treated with the AP. Mean time from in domo imaging to groin puncture, time from groin puncture to first pass with first flow restoration and time from groin to final successful recanalization was not statistically different between both groups (Table 2).
Safety
There was a significant difference in the rate of postinterventional hemorrhage and the stent-retriever used to perform MT, as postinterventional hemorrhage defined according to morphological classification of ECASS and HBC was less frequent in the APH group. Moreover, subarachnoid hemorrhage (HBC 3c) was significantly less frequent in the APH group (44/148, 29.7% vs. 24/150, 16.0%, p = 0.005). There was no statistical difference between both groups regarding clinical deterioration of ≥ 4 points on the NIHSS concomitant with either evidence of any form of postinterventional hemorrhage (as defined in ECASS II), evidence of PH2 (ECASS) or HBC type 2 hemorrhage (as defined in SITS-MOST) or evidence of subarachnoid hemorrhage (sSAH). In domo mortality of patients treated with the new generation APH was slightly higher (21/148, 14.2% vs. 28/150, 18.7%) but without significant difference (Table 3).
Discussion
In this study both the first generation APERIO® (AP) and its successor, the new generation APERIO® Hybrid (APH) stent-retriever device achieved similarly high rates of successful recanalization (eTICI ≥ 2b67: 75.7% for AP vs. 79.3% for APH) according to the definition by Liebeskind et al. 2019 [21]. Full recanalization, defined as eTICI ≥ 2c, could be achieved in 46.7% for AP vs. 56.0% for APH. These observations did not significantly differ between the two devices but were nominally lower in the AP group. In both groups a median of 2 (IQR 1–3) passes were required for recanalization. Additionally, there were few more cases in the APH group (8 (5.3%) vs. 2 (1.4)) where successful recanalization could not be achieved despite using various bail-out devices. Hence, in these cases we believe the lack of successful recanalization cannot be related to the stent-retriever that has been employed.
Considering the different definitions of successful recanalization, our primary measures of effectiveness fall into comparable ranges of external estimates reported by large prospective registry studies like the North American STRATIS registry (87.9% full recanalization/mTICI ≥ 2b, median (IQR) of 1 (1–2) and 2 (1–3) passes, depending on stent-retriever size), the TRACK registry (80.3% full recanalization/mTICI ≥ 2b, mean and SD of 1.9 ± 1.2 passes) or the German Stroke Registry (83.0% full recanalization/mTICI ≥ 2b; median and IQR of 2 (1–3) passes) [24,25,26,27,28]. In view of our observations and their comparison with external estimates, both devices can be considered effective to achieve successful recanalization. This interpretation is further supported by the observation that in this study only 5 LVO (3.3%) in the APH group and only 3 LVO (2.0%) in the AP group eventually could not be recanalized using these stent-retriever as first choice but could be recanalized after switching to other types of stent-retriever.
In terms of safety, we looked at hemorrhagic complications after MT and focused on the hemorrhagic entity we considered to be possibly associated with the mechanical impact of the stent-retriever on the vessel wall: subarachnoid hemorrhage in spatial association with the site of mechanical endovascular manipulation. Regardless of the clinical neurological relevance of intracranial hemorrhage, there was a significant difference of the rates of postinterventional hemorrhage within 24 h between both groups as any intracranial hemorrhage occurred less frequently in the APH group. Interestingly, any signs of potentially device-attributable subarachnoid hemorrhage were in fact detected less frequently for the new generation APH device (24/150, 16.0%) compared with its predecessor (44/148, 39.7%). Obviously, it is of importance to include the clinical/neurological course of the patient when evaluating postinterventional hemorrhagic sequelae. To this end, there are various previously published definitions of symptomatic intracranial hemorrhage (sICH). In order to ensure proper comparability, we applied these pre-existing definitions of sICH also to our groups. According to the ECASS II protocol sICH is defined as any hemorrhage detected after MT that is associated with clinical deterioration of ≥ 4 points on NIHSS [19]. In SITS-MOST only parenchymal hematoma defined as PH2 according to ECASS or type 2 according to HBC, associated with clinical deterioration of ≥ 4 points on the NIHSS was defined as sICH [20]. By applying these two definitions of sICH we observed no significant differences between both groups. The prevalence of postinterventional sICH according to ECASS II was relatively high in our study (16.9% for AP vs. 14.7% for APH), but it needs to be acknowledged that the rates of hemorrhagic complications may be fairly overestimated using this definition, as a causal relationship between hemorrhagic transformation without mass-effect and clinical deterioration can only vaguely be inferred. By applying the sICH definition according to SITS-MOST, we observed considerably lower rates of sICH for both groups (3.4% for AP vs 0.7% for APH, p = 0.096). Although there was no statistically significant difference between both groups, sICH was remarkably low in the group treated with the new generation APH. Our sICH rates are in a range that is comparable to other external estimates. The TRACK registry reported rates of sICH (defined as any parenchymal, subarachnoid or intraventricular hemorrhage associated with worsening of the NIHSS by ≥ 4 points within 24 h) between 4.5–9.8%, depending on volume of stroke centers [29]. In the STRATIS registry the rate of sICH (defined as any PH1, PH2, remote intraparenchymal hemorrhage or intraventricular hemorrhage associated with worsening of the NIHSS by ≥ 4 points within 24 h) was reported to be 1.4% [26] and in a meta-analysis of the HERMES collaboration Goyal et al. reported 4.4% of postinterventional sICH (as defined by each trial) [3]. As we mentioned before, in our attempt to associate the occurrence of CT signs of hemorrhage to stent-retriever type, we had to define a novel type of sICH: CT detection of subarachnoid hemorrhage on postinterventional CT with concomitant clinical worsening of ≥ 4 points on NIHSS within 24 h (=sSAH). By applying this definition, we found no significant difference between both groups (11.5% for AP vs. 8.5% for APH). These results suggest that the new generation APH is efficient and safe in comparison to its predecessor with a trend towards improved safety with respect to hemorrhagic complications; however, the finding of the aforementioned difference in the detection of hemorrhage between groups may also represent the potential bias that considerably more patients of the AP group required stenting for tandem stenosis of the internal carotid artery. This usually resulted in double platelet inhibition immediately or soon after the intervention and hence leads to an increased risk of hemorrhage especially into predamaged ischemic infarction tissue, which we believe is independent from the stent retriever device used. Subjective ratings from interrogating four participating neurointerventionalists indicated that tractability was equally “good” or “very good” for both devices while visibility of the APH was rated as “very good” by all respondents and was improved compared to its predecessor. The substantially improved visibility of the new generation APH may also be at least partly held accountable for this favorable evolution of hemorrhagic risk to a very low level over time in this study, as it allows better control over the retrieval maneuver in the most critical moments of MT and substantially better visibility. Any potential bias underlying this interpretation cannot be completely resolved by this study. The observed estimates of bleeding type frequency that we found per group are descriptive in that these results cannot prove or disprove direct or indirect cause association with the stent-retriever type; however, with the intent of comparing two groups (novel device generation vs. predecessor generation) we found no evidence of substantial differences between bleeding type frequency, which supports the clinically relevant conclusion that the novel device type is equally safe with respect to bleeding complications that were comprehensively classified and evaluated with particular detail for the purpose of this study.
In domo mortality was at 14.2% for the AP group and at 18.7% for the APH group with the limitation that we did not collect any follow-up data after discharge. Therefore, the comparability with the observations of other studies and current registries is limited in this respect (mortality: STRATIS 14.4%, TRACK 19.8% and German Stroke Registry 28.5%) [24, 26, 27].
The observation of marginally fewer hemorrhagic complications in the group treated with the new generation APH did not result in a better early clinical outcome as assessed by the NIHSS at follow-up (24/38/72 h) and in-hospital mortality; however, the higher proportion of patients with basilar artery occlusion in the APH group must also be considered as a potential bias, as it has been described as an independent predictor of unfavorable outcome [30].
Procedural time metrics were not different between both stent-retriever groups, indicating that handling and navigability of the new generation APH is not inferior to the AP device. This comprises the median time interval from groin to first pass, which was slightly but not significantly shorter for the AP (33 min vs. 41 min) and which also was within a range that is comparable to other studies outside of the randomized controlled intervention trials. Pfaff et al., for example, reported a median of 35 min from groin to first flow restoration observing a new generation stent-retriever from another manufacturer [31]. There was also no statistical difference in the time from groin to final recanalization or in the time from groin to first pass.
In an already published early experience multicenter study of 48 patients, the APH stent-retriever device has been described as feasible, efficient and safe by Kaschner et al. [32]. Our study adds to this previous evidence by evaluating a substantially larger cohort and, in particular, providing an equally large and relatively well-balanced reference group treated with the previous model of the APERIO® stent-retriever device. We consider as strengths of our study that a relatively large number of patients could be observed and that both groups were similar in size and regarding baseline characteristics. For these reasons, we believe that despite its retrospective design, this study does not preclude the clinically relevant interpretations. At the same time, we acknowledge that the risk of any bias including also unidentified factors is inherently higher through retrospective observation. This includes also a possible increase of experience over time and its potential impact on the reported complication rates; however, by accurately reporting procedural time intervals we can provide one of the most important surrogate measures of operator experience. These measures suggest that there do not seem to be any relevant differences in operator experience. Arguably the most relevant strength of this study was that the transition between first and successive next generation devices was chronological at our institution with only a short period of negligible temporal overlap where both devices were available.
Conclusion
The new generation APERIO® Hybrid thrombectomy device was observed to be efficient and safe in direct comparison to its predecessor. In addition, postinterventional hemorrhage, and particularly local subarachnoid hemorrhages as a bleeding entity possibly associated with the stent-retriever device occurred less frequently, which could be, at least in part, related to refinements of stent-retriever technology and improved handling and visibility of the new generation device.
References
Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, Schellinger PD, Toni D, de Vries J, White P, Fiehler J. European Stroke Organisation (ESO)- European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2019;11:535–8.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–418. Erratum in: Stroke. 2019;50:e440–1.
Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millán M, Davis SM, Roy D, Thornton J, Román LS, Ribó M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–31.
Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, Millán M, Urra X, Cardona P, López-Cancio E, Tomasello A, Castaño C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez-Pérez M, Goyal M, Demchuk AM, von Kummer R, Gallofré M, Dávalos A; REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–306.
Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–95.
Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama à Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. Erratum in: N Engl J Med. 2015;372:394.
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30.
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18.
Turk AS 3rd, Siddiqui A, Fifi JT, De Leacy RA, Fiorella DJ, Gu E, Levy EI, Snyder KV, Hanel RA, Aghaebrahim A, Woodward BK, Hixson HR, Chaudry MI, Spiotta AM, Rai AT, Frei D, Almandoz JED, Kelly M, Arthur A, Baxter B, English J, Linfante I, Fargen KM, Mocco J. Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet. 2019;393:998–1008.
Lapergue B, Blanc R, Gory B, Labreuche J, Duhamel A, Marnat G, Saleme S, Costalat V, Bracard S, Desal H, Mazighi M, Consoli A, Piotin M; ASTER Trial Investigators. Effect of Endovascular Contact Aspiration vs Stent Retriever on Revascularization in Patients With Acute Ischemic Stroke and Large Vessel Occlusion: The ASTER Randomized Clinical Trial. JAMA. 2017;318:443–52.
Bayerisches Landesamt für Statistik. Regionalisierte Bevölkerungsvorausberechnung für Bayern bis 2038. 2020.
Lyden PD, Lu M, Levine SR, Brott TG, Broderick J; NINDS rtPA Stroke Study Group. A modified National Institutes of Health Stroke Scale for use in stroke clinical trials: preliminary reliability and validity. Stroke. 2001;32:1310–7.
Pexman JH, Barber PA, Hill MD, Sevick RJ, Demchuk AM, Hudon ME, Hu WY, Buchan AM. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001;22:1534–42.
Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132:2231–8.
Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, Sacks D; Technology Assessment Committee of the American Society of Interventional and Therapeutic Neuroradiology; Technology Assessment Committee of the Society of Interventional Radiology. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–37. Epub 2003 Jul 17. Erratum in: Stroke. 2003 Nov;34(11):2774.
Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, Marks MP, Prabhakaran S, Kallmes DF, Fitzsimmons BF, Mocco J, Wardlaw JM, Barnwell SL, Jovin TG, Linfante I, Siddiqui AH, Alexander MJ, Hirsch JA, Wintermark M, Albers G, Woo HH, Heck DV, Lev M, Aviv R, Hacke W, Warach S, Broderick J, Derdeyn CP, Furlan A, Nogueira RG, Yavagal DR, Goyal M, Demchuk AM, Bendszus M, Liebeskind DS; Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization working group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–63.
von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, Treurniet KM, Majoie CB, Marquering HA, Mazya MV, San Román L, Saver JL, Strbian D, Whiteley W, Hacke W. The Heidelberg Bleeding Classification: Classification of Bleeding Events After Ischemic Stroke and Reperfusion Therapy. Stroke. 2015;46:2981–6.
Larrue V, von Kummer R R, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke. 2001;32:438–41.
Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, Schneider D, Diez-Tejedor E, Trouillas P. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–51.
Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, Lees KR, Roine RO, Soinne L, Toni D, Vanhooren G; SITS-MOST investigators. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275–82. Erratum in: Lancet. 2007;369:826.
Liebeskind DS, Bracard S, Guillemin F, Jahan R, Jovin TG, Majoie CB, Mitchell PJ, van der Lugt A, Menon BK, San Román L, Campbell BC, Muir KW, Hill MD, Dippel DW, Saver JL, Demchuk AM, Dávalos A, White P, Brown S, Goyal M; HERMES Collaborators. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019;11:433–8.
Humphries W, Hoit D, Doss VT, Elijovich L, Frei D, Loy D, Dooley G, Turk AS, Chaudry I, Turner R, Mocco J, Morone P, Fiorella D, Siddiqui A, Mokin M, Arthur AS. Distal aspiration with retrievable stent assisted thrombectomy for the treatment of acute ischemic stroke. J Neurointerv Surg. 2015;7:90–4.
Maus V, Henkel S, Riabikin A, Riedel C, Behme D, Tsogkas I, Hesse AC, Abdullayev N, Jansen O, Wiesmann M, Mpotsaris A, Psychogios MN. The SAVE Technique : Large-Scale Experience for Treatment of Intracranial Large Vessel Occlusions. Clin Neuroradiol. 2019;29:669–76.
Wollenweber FA, Tiedt S, Alegiani A, Alber B, Bangard C, Berrouschot J, Bode FJ, Boeckh-Behrens T, Bohner G, Bormann A, Braun M, Dorn F, Eckert B, Flottmann F, Hamann GF, Henn KH, Herzberg M, Kastrup A, Kellert L, Kraemer C, Krause L, Lehm M, Liman J, Lowens S, Mpotsaris A, Papanagiotou P, Petersen M, Petzold GC, Pfeilschifter W, Psychogios MN, Reich A, von Rennenberg R, Röther J, Schäfer JH, Siebert E, Siedow A, Solymosi L, Thonke S, Wagner M, Wunderlich S, Zweynert S, Nolte CH, Gerloff C, Thomalla G, Dichgans M, Fiehler J. Functional Outcome Following Stroke Thrombectomy in Clinical Practice. Stroke. 2019;50:2500–6.
Flottmann F, Brekenfeld C, Broocks G, Leischner H, McDonough R, Faizy TD, Deb-Chatterji M, Alegiani A, Thomalla G, Mpotsaris A, Nolte CH, Fiehler J, Maros ME; GSR investigators*. Good Clinical Outcome Decreases With Number of Retrieval Attempts in Stroke Thrombectomy: Beyond the First-Pass Effect. Stroke. 2021;52:482–90.
Kallmes DF, Baxter BW, Jumaa MA, Sunenshine P, Majjhoo A, English JD, Suzuki S, Fessler RD, Delgado Almandoz JE, Martin JC, Haussen DC; STRATIS Investigators. Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischemic Stroke: Primary Results of the STRATIS Registry. Stroke. 2017;48:2760–8.
Zaidat OO, Castonguay AC, Nogueira RG, Haussen DC, English JD, Satti SR, Chen J, Farid H, Borders C, Veznedaroglu E, Binning MJ, Puri A, Vora NA, Budzik RF, Dabus G, Linfante I, Janardhan V, Alshekhlee A, Abraham MG, Edgell R, Taqi MA, Khoury RE, Mokin M, Majjhoo AQ, Kabbani MR, Froehler MT, Finch I, Ansari SA, Novakovic R, Nguyen TN. TREVO stent-retriever mechanical thrombectomy for acute ischemic stroke secondary to large vessel occlusion registry. J Neurointerv Surg. 2018;10:516–24.
Zaidat OO, Haussen DC, Hassan AE, Jadhav AP, Mehta BP, Mokin M, Mueller-Kronast NH, Froehler MT. Impact of Stent Retriever Size on Clinical and Angiographic Outcomes in the STRATIS Stroke Thrombectomy Registry. Stroke. 2019;50:441–7.
Nogueira RG, Haussen DC, Castonguay A, Rebello LC, Abraham M, Puri A, Alshekhlee A, Majjhoo A, Farid H, Finch I, English J, Mokin M, Froehler MT, Kabbani M, Taqi MA, Vora N, Khoury RE, Edgell RC, Novakovic R, Nguyen T, Janardhan V, Veznedaroglu E, Prabhakaran S, Budzik R, Frankel MR, Nordhaus BL, Zaidat OO. Site Experience and Outcomes in the Trevo Acute Ischemic Stroke (TRACK) Multicenter Registry. Stroke. 2019;50:2455–60.
Alonso de Leciñana M, Kawiorski MM, Ximénez-Carrillo Á, Cruz-Culebras A, García-Pastor A, Martínez-Sánchez P, Fernández-Prieto A, Caniego JL, Méndez JC, Zapata-Wainberg G, De Felipe-Mimbrera A, Díaz-Otero F, Ruiz-Ares G, Frutos R, Bárcena-Ruiz E, Fandiño E, Marín B, Vivancos J, Masjuan J, Gil-Nuñez A, Díez-Tejedor E, Fuentes B; Madrid Stroke Network. Mechanical thrombectomy for basilar artery thrombosis: a comparison of outcomes with anterior circulation occlusions. J Neurointerv Surg. 2017;9:1173–8.
Pfaff J, Rohde S, Engelhorn T, Doerfler A, Bendszus M, Möhlenbruch MA. Mechanical Thrombectomy Using the new Solitaire™ Platinum Stent-retriever : Reperfusion Results, Complication Rates and Early Neurological Outcome. Clin Neuroradiol. 2019;29:311–9.
Kaschner M, Lichtenstein T, Weiss D, Turowski B, Goertz L, Kluner C, Schlamann M, Mathys C, Kabbasch C. The New Fully Radiopaque Aperio Hybrid Stent Retriever: Efficient and Safe? An Early Multicenter Experience. World Neurosurg. 2020;141:e278–88.
Funding
Project grant by Acandis® GmbH. Acandis® company had no role in the design, conduct or interpretation of the study and no access to study data.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
M.L.V., A.M.K., M.P. and A.M. contributed to the study design and data analyses. Data collection was performed by M.L.V., A.M.K., A.M., F.W., M.S., J.F and FE. Rating of hemorrhagic complications was performed by A.M. and M.S. The first draft of the manuscript was written by M.L.V. and all authors commented on previous versions of the manuscript. All authors provided important intellectual content and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
M.L. Vogt, A.M. Kollikowski, F. Weidner, M. Strinitz, J. Feick, F. Essig, H. Neugebauer, K.G. Haeusler, M. Pham and A. Maerz declare that they have no competing interests.
Ethical standards
This retrospective study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The institutional ethics committee of Julius-Maximilians-University Würzburg, Germany approved this study. Informed consent was waived by the institutional ethics committee due to the retrospective nature of this study. Consent to participate: not applicable. Consent for publication: not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vogt, M.L., Kollikowski, A.M., Weidner, F. et al. Safety and Effectiveness of the New Generation APERIO® Hybrid Stent-retriever Device in Large Vessel Occlusion Stroke. Clin Neuroradiol 32, 141–151 (2022). https://doi.org/10.1007/s00062-021-01122-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-021-01122-1