Abstract
Purpose
This study aimed to evaluate the effectiveness and safety of the NeVaTM stent retriever as first- and second-line device for mechanical thrombectomy in acute ischemic stroke.
Methods
In this retrospective single-center study, all consecutive patients that underwent mechanical thrombectomy with NeVaTM stent retriever as first- or second-line device due to intracranial vessel occlusion with acute ischemic stroke between March and November 2022 were included.
Results
Thirty-nine patients (m=18, f=21) with a mean age of 69.9 ± 13.3 years were treated with the NeVaTM stent retriever. NeVaTM stent retriever was used as first-line device in 24 (61.5%) of patients and in 15 (38.5%) as second-line device. First-pass rate (≥mTICI 2c) of NeVaTM stent retriever was both 66.7% when used as first- or second-line device. Final recanalization rate including rescue strategies was 92.3% for ≥mTICI2c and 94.9% for ≥mTICI2b. No device-related minor or major adverse events were observed. A hemorrhage was detected in 33.3% of patients at 24h post-thrombectomy dual-energy CT, of which none was classified as symptomatic intracerebral hemorrhage. NIHSS and mRS improved significantly at discharge compared to admission (p<0.05).
Conclusion
The NeVaTM stent retriever has a high effectivity and good safety profile as first- and second-line device for mechanical thrombectomy in acute ischemic stroke.
Similar content being viewed by others
Introduction
Mechanical thrombectomy (MT) based on clot-retrieval by stent retrievers, perceived as the most effective devices for fast and safe recanalization superior to intravenous thrombolysis alone, has emerged as standard care for patients with acute ischemic stroke (AIS) [1,2,3,4,5]. MT aims to accomplish complete reperfusion of occluded vasculature; herein, the achieved grade of recanalization is measured by the modified Thrombolysis in Cerebral Infarction (mTICI) Score. Successful recanalization defined as mTICI≥2b as a measure for technical success was used in the majority of studies [6]. Meanwhile, it has been shown that higher reperfusion rates and especially complete recanalization (mTICI 3) lead to improved clinical outcome [7]. Fast recanalization as measured by the first-pass rate (complete reperfusion ≥mTICI 2c with a single pass of a stent retriever) has also been shown to be associated with significantly higher rates of good clinical outcome and is therefore used to evaluate the performance of new devices [8,9,10]. In recent years, various new stent retrievers with different size, shapes, and materials have been introduced. While the choice of material is currently up to the neurointerventionalists’ discretion depending on the individual case, evidence-based decision-making should be fostered by future research. In this context, it has been shown for some devices that a larger diameter or longer size of the stent retriever may improve successful first-pass rate and final mTICI score in large vessel occlusion [11, 12]. However, MT may still remain challenging especially with organized or hard, fibrin-rich, and sticky clots. The NeVaTM stent retriever device has recently been designed with multifunctional drop zones and high radial force to improve first-pass rate for all clot types. The NeVaTM stent retriever device showed promising results in first preclinical [13, 14] and clinical studies [15,16,17,18,19], but evidence for first- and especially second-line use of this device is still limited.
The aim of this study was therefore to evaluate the effectiveness and safety of the NeVaTM stent retriever as first- and second-line device for mechanical thrombectomy in acute ischemic stroke.
Methods and material
Study design
This retrospective study included all consecutive patients with acute ischemic stroke (AIS) who received mechanical thrombectomy (MT) with the use of the NeVaTM 4 × 30-mm stent retriever (Vesalio LLC, Nashville, USA) in a tertiary stroke center between March and November 2022. The study was approved by the local ethics committee. Informed patient consent was waived due to the retrospective character of this study.
Diagnosis of AIS was made by computed tomography (CT), CT angiography (CTA), and CT perfusion (CTP), if patient presented in-house. Patients transferred from external hospitals were examined there by CT or magnetic resonance imaging (MRI) and referred to the study center depending on imaging results and supply capacity. Additional intravenous lysis therapy was performed immediately after diagnosis in case of no contraindications. Neurological assessments were performed by trained neurologists in a tertiary stroke center. Decision for mechanical thrombectomy was made according to national guidelines [20] and in consensus by interventional neuroradiologist and neurologist on service, having at least 5 years of professional experience in stroke care.
Baseline data of the study cohort were retrieved from the Clinical Information System (CIS) and the radiology information system (RIS) as well as the Picture Archiving and Communication System (PACS) regarding:
• Patient characteristics (sex; age)
• Baseline clinical parameters (modified Rankin scale (mRS); National Institutes of Health Stroke Scale (NIHSS); etiology of stroke; preprocedural intravenous lysis therapy; time from symptom onset to femoral puncture)
• Baseline imaging characteristics (location and side of vessel occlusion)
Mechanical thrombectomy: technique and technical outcome evaluation
MT was performed by trained and certified interventional neuroradiologists with at least 5 years of professional experience. In all cases, MT was performed via an arterial transfemoral approach in general anesthesia. This retrospective study included patients with use of NeVaTM stent retriever as first- and second-line device for MT. Thus, before- or afterhand use of other stent retriever devices was not an exclusion criterion. The choice to use NeVaTM stent retriever as first- or second-line device was left to the discretion of the neurointerventionalist performing the procedure and depended on the location of the thrombus as well as underlying vessel size and configuration. The NeVaTM stent retriever has been described in detail elsewhere [13, 16]. Briefly, the device is a novel hybrid-cell, multizone stent retriever consisting of two large open areas ensuring entry points for clots as well as a closed-ended basket-shape zone at the distal end retaining entrapped thrombus (Fig. 1). The design is supposed to retrieve both CT hypodense (Fig. 2a–f) and CT hyperdense, fibrin-rich/calcified thrombus (Fig. 2g–k). In all cases, either an 8F balloon-guided catheter (FlowGate, Stryker, Kalamazoo, USA) or 6F long sheath (NeuronMax, Penumbra, Alameda, USA) was placed in the internal carotid artery, connected with a continuous flush line charged with nimodipine (2 mg/l in saline). Intermediate catheters, such as ACE68, JET7, 5MAX (Penumbra, Alameda, USA) or 5F Sofia, and REACT68 (Medtronic, Irvine, USA), were also used at the discretion of the performing neurointerventionalist. A microcatheter (Rebar18, Medtronic, Irvine, USA) was placed distal to the occluding thrombus and the stent retriever deployed. Withdrawal of the stent retriever was performed under suction by a 50cc syringe or electric aspiration pump at the guiding catheter and, if used, the intermediate catheter.
Design of NeVaTM stent retriever. Illustration of the NeVaTM stent retriever (4 × 30 mm) consisting of two drop zones (blue bold arrows) labeled by radiopaque markers (blue dotted arrows). The drop zones are designed as entry points for clots. The closed-ended basket-shape zone at the distal end is supposed to retain entrapped thrombus (photograph provided by Vesalio LLC, Nashville, USA)
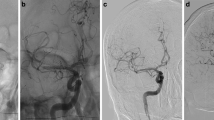
Exemplary case examples of mechanical thrombectomy with NeVaTM stent retriever in mechanical thrombectomy with different thrombus morphology. a Axial CT and b coronal CT angiography of a patient with acute ischemic stroke due to hypodense thrombus (blue circle) in M1 segment of the right middle cerebral artery. c Digital substraction angiography (DSA) showed right M1 occlusion. d Placement of NeVaTM stent retriever at the site of M1 occlusion, radiopaque markers (proximal, distal, and drop zones (white arrows)) are clearly depicted. e DSA after one pass with NeVaTM stent retriever showed complete recanalization of the previously occluded segment. f Histology of retrieved thrombus showed mainly erythrocyte-rich areas with only some fibrin and calcifications, correlating with CT findings. g Axial CT and h coronal CT angiography of a patient with acute ischemic stroke due to hyperdense, calcified thrombus (blue circle) in M1 segment of the left middle cerebral artery. i Digital substraction angiography (DSA) showed left M1 occlusion. j DSA after one pass with NeVaTM stent retriever showed complete recanalization of the previously occluded segment. k Photograph of a thrombus of a different patient retrieved by NeVaTM stent retriever showing entrapped thrombus at distal end of the device. l In contrast to patient a–f histology of the thrombus retrieved from the patient g–j showed a heavily fibrin-rich, low in erythrocytes thrombus with some calcifications (black arrow) correlating with CT data. As shown by patients a–f and g–l, NeVaTM showed successful first-pass recanalization in both types of thrombus causing acute cerebral ischemia
Procedural data analysis included if the procedure was performed with or without balloon-guided catheter as well as with or without intermediate catheter, usage of the NeVaTM stent retriever as first- or non-first-line device, rate of stent retriever changes from NeVaTM to other stent retrievers and vice versa, and number of passes with NeVaTM stent retriever.
Technical outcome evaluation was based on the Modified Thrombolysis in Cerebral Infarction (mTICI) [21] score with NeVaTM first-pass ≥mTICI 2c and ≥mTICI 2b recanalization rate and final mTICI score. Technical outcome evaluation was performed by board certified radiologists with at least 5 years of professional experience in stroke care.
Mechanical thrombectomy: safety evaluation
All procedural data and patient recordings were analyzed with regard to any device-related minor or major adverse events. Further, postinterventional dual-energy CT data performed 24 h after MT were reviewed for any intracranial hemorrhage. Symptomatic intracerebral hemorrhage (SICH) was defined based on ECASS-III (European Cooperative Acute Stroke Study) criteria as any intracranial hemorrhage associated with neurological deterioration of ≥4 points on the NIHSS score at 24 h [22]. Safety outcome evaluation was performed by board certified radiologists (CT) and stroke neurologists (SICH) with at least 5 years of professional experience in stroke care.
Clinical outcome evaluation
Clinical outcome after MT was based on the extent of neurological impairment as assessed by NIHSS at 24h after MT as well as NIHSS and mRS at the time of discharge. Neurological assessments for clinical outcome evaluation were performed by trained stroke neurologists of a tertiary care stroke center.
Statistical analysis
Statistical analyses were performed using SPSS version 28.0.1 (SPSS Inc., Chicago, IL, USA). All data are presented as the mean (±SD), median (range), absolute, or percentage depending on nature of variables and distribution. Chi-square test was used for contingency tables. For analysis of any parameters associated with a successful first-pass recanalization of the NeVaTM stent retriever (≥mTICI 2c), a binary logistic regression was used. For comparison of NIHSS at admission, 24h after MT and at discharge a Friedman test for non-parametric paired data with respective post hoc test was used. For comparison of mRS score at admission and discharge, a paired t-test was used. Two-sided p-values < 0.05 were defined as statistically significant.
Results
Study cohort
This study included 39 patients (18 male, 21 female) with a mean age of 69.9 (± 13.3, range 26–88) years who received a mechanical thrombectomy with NeVaTM stent retriever, selected from a total cohort of 212 patients receiving mechanical thrombectomy within the study period. A total of 25 (64.1%) patients presented with vascular occlusion of the M1 segment of the middle cerebral artery (MCA-M1), 7 (17.9%) patients with occlusion of the M2 segment of the middle cerebral artery (MCA-M2), 3 (7.7%) patients presented with a combined occlusion of the internal carotid artery (ICA) and an intracranial vessel (2× M1, 1× M2), and 4 (10.3%) patients presented with occlusion of the basilar artery (BA). Vessel occlusions of anterior circulation were located on the right side in 22 (56.4%) and on the left side in 13 (33.3%) of patients. Systemic lysis therapy was performed in 15 (38.5%) patients. The mean time from onset of symptoms to femoral puncture was 297.8 ± 203.6 min. Etiology of stroke was cardioembolic in 12 (30.8%) patients, thrombotic due to local stenosis in 2 (5.1%) patients, due to aortic valve endocarditis in 1 (2.6%) patient, embolic after thoracic surgery in 1 (2.6%) patient, due to drug abuse in 1 (2.6%) patient, and unknown in 22 (56.4%) patients.
Detailed patient characteristics are shown in Table 1.
Mechanical thrombectomy: procedural characteristics and technical outcome evaluation
Detailed information on procedural characteristics and technical outcome are shown in Table 2 and recanalization rates are also illustrated in Fig. 3. The NeVaTM stent retriever was used as first-line thrombectomy device in 24 (61.5%) patients and in 15 (38.5%) patients as second-line device. A balloon-guided catheter was used in 30 (76.9%) patients; an intermediate catheter was used in 13 (33.3%) patients. Additional stenting was performed in 4 (10.3%) patients due to underlying stenosis (75%) or dissection (25%, not NeVaTM stent retriever related).
mTICI scores of first- or second-line NeVaTM stent retriever use. mTICI scores 1) after NeVaTM stent retriever as first-line device 2), after NeVaTM stent retriever as first-line device + additional maneuvers (ADM; other devices, rescue etc.) 3), before NeVaTM stent retriever as second-line device 4), after NeVaTM stent retriever as second-line device, and 5) final mTICI score of the entire cohort
When NeVaTM stent retriever was used as first-line device (24/39 patients), a mean of 1.2±0.4 passes was performed. Here, a first-pass rate mTICI ≥ 2c of 66.7% (16/24 patients) was observed. After first-line use of NeVaTM stent retriever, a rate of mTICI≥ 2b of 91.7% (22/24) and of mTICI≥2c of 18/24 (75%) was achieved. Successful recanalization ≥ mTICI 2c was achieved in all (16/16, 100%) procedures, where only the NeVaTM stent retriever and no other device has been used.
Additional maneuvers with a different stent retriever to further improve recanalization rate were performed at the discretion of the performing neurointerventionalist in 7/24 (29.1%) patients with a mean of 1.7±0.9 additional passes. Those additional stent retrievers were mainly used for the remaining peripheral vessel occlusions (≥ distal M2) after partial recanalization (4/7, 57.1%), while they were used for remaining main vessel occlusion in 2/7 (28.6%) and in 1/7 (14.2%) for peripheral vessel occlusion (A3) due to embolization to new territory. Final mTICI rate after first-line NeVaTM stent retriever and additional maneuvers was mTICI ≥ 2c of 100% (24/24).
NeVaTM stent retriever was used as second-line device for mechanical thrombectomy in 15/39 (38.5%) patients. A mean number of 2.1±1.7 passes were performed with other stent retrievers afore NeVaTM stent retriever was used. Here, in 6/15 patients a pRESET 6 × 50 mm (phenox, Bochum, Germany) was used before usage of NeVaTM stent retriever, while in 3/15 patients a pRESET 6 × 50 mm and a CatchView 5.5 × 50 mm (balt, Montmorency, France), in 2/15 patients a CatchView 5.5 × 50 mm, in 1/15 patient a pRESET 6 × 50 mm and a pRESET 4 × 20 mm, in 1/15 patient a pRESET 6 × 30 mm, in 1/15 patients a pRESET 6 × 30 and a CatchView 5.5 × 50 mm, and in 1/15 patients a pRESET 4 × 20 mm stent retriever was used. Before usage of NeVaTM stent retriever, the rate of mTICI ≥2b/2c was 6.7% (1/15, second-line NeVaTM stent retriever was used for remaining M2 in this case) with all other patients showing mTICI ≤ 2a (mTICI 0: 9; mTICI 1: 3; mTICI 2a: 2).
A mean of 1.7±1.4 passes with NeVaTM stent retriever as second-line device was performed, showing a first-pass rate mTICI ≥2c of 66.7% (10/15 patients). Herewith, the rate of mTICI ≥2b improved to 86.7 % (13/15) and of mTICI ≥ 2c to 80% (12/15). The two patients still showing mTICI <2b after second-line NeVaTM maneuvers were both also not recanalizable with any other additional maneuvers/devices (final mTICI score 0 and 1, respectively). No embolization to new territory was observed in the second-line group.
Summarizing the entire cohort (39 patients) with first- or second-line use of NeVaTM stent retriever, a total of 2.9±3.0 passes (median 2, range 1–16), of which 1.4±0.9 passes (median 1, range 1-5) were with NeVaTM stent retriever, were performed. NeVaTM stent retriever first-pass rate mTICI ≥2c was 66.7% (26/39). After thrombectomy with NeVaTM stent retriever first- or second-line and additional maneuvers, a rate of mTICI ≥2b of 94.9% (37/39) and of mTICI ≥2c of 92.3% (36/39) was observed.
There were neither any baseline patient characteristics (age, sex, mRS, NIHSS, location, side or number of vessel occlusions, etiology of stroke) nor any procedural parameters (systemic i.v. lysis prior to thrombectomy, use of balloon-guided or intermediate catheter, NeVaTM stent retriever as first- or second-line device) significantly associated with a successful first-pass recanalization of the NeVaTM stent retriever (all p-values >0.05).
Mechanical thrombectomy: safety evaluation
No minor or major adverse events were observed in the study cohort. Only 1 patient (2.6%) showed NeVaTM stent retriever-associated intracranial vasospasm after thrombectomy, which fully resolved after short increase in running rate of catheter flushing solution containing nimodipine. A total of 13/39 (33.3%) patients showed a hemorrhage at 24h postprocedural CT scan, of which none was a symptomatic intracerebral hemorrhage (SICH) as defined by worsening of the NIHSS of at least four points. In these cases, vessel occlusion was located in 9/13 (69.2%) within the M1-segment, in 3/13 (23.1%) patients within the M2-segment and in 1/13 (7.7%) within the ICA and M1-segment. We have added this information to the results section of the manuscript. Overall in-hospital mortality rate was 4/39 (10.3%) patients.
Clinical outcome evaluation
NIHSS score showed significant differences at admission, 24h after MT, and at discharge as revealed by the Friedman test (X2=22.4, p<0.001). Here, Friedman post hoc test showed that NIHSS was significantly lower at 24h after MT (13.1±12.1, p=0.008) and at discharge (11.2±12.7, p<0.001) than at admission (13.9±6.8). Meanwhile, NIHSS was not significantly different at 24h after MT compared to discharge (p=0.141). mRS improved significantly from 4.6±0.8 at admission to 3.3±2.0 at discharge (p<0.001). mRS 0–2 was reached in 14/39 (35.9%) of patients at discharge.
Discussion
This study shows high technical effectiveness and safety profile of the NeVaTM stent retriever for mechanical thrombectomy in acute ischemic stroke. In detail, the first-pass rate (recanalization ≥ mTICI 2c) of the NeVaTM stent retriever was observed with 66.7%, which is higher than reported for other stent retriever devices (~22–40%) [23,24,25,26,27,28]. Since successful first-pass recanalization is associated with a better and an increasing number of passes is associated with a worse outcome [9, 23], this is an important device-related measure most probably explainable by the high radial force and the drop-zone design of the NeVaTM stent retriever. First-pass rate was also higher than reported for the initial experiences with the NeVaTM stent retriever reporting a first-pass rate of 43.9–48.3% [15,16,17, 19] and more similar to the up to date largest cohort reporting a rate of 53.8% in first-line treatment with NeVaTM stent retriever [18]. To the best of our knowledge, this is the first study also reporting effectivity of the NeVaTM stent retriever as second-line device. Here, first-pass rate was as well 66.7% after various other stent retrievers failed to recanalize after a mean of 2.1±1.7 passes emphasizing the potential of the NeVaTM stent retriever as an effective rescue device. This notion should be further evaluated in future prospective studies including a larger cohort of patient with second-line use of the NeVaTM stent retriever.
On the other hand, our study reports the need for other devices after first-line NeVaTM stent retriever in 29.1% of cases, which is higher (7.6–13.8%) [15, 16], than reported in other studies on other devices. This is best explained due to a lower number of passes performed with NeVaTM stent retriever especially when used as first-line device compared to elsewhere indicating an earlier decision towards a change of device [15,16,17]. Further, this can be explained by a high rate (57.1%) of other devices needed only for remaining peripheral vessel occlusions (≥distal M2). However, a contribution of partial clot retraction with consequent distal emboli cannot be fully excluded here.
Nevertheless, a preferably high recanalization rate should be the goal of MT also beyond the first-pass effect [26]. Here, final recanalization mTICI ≥2b including rescue strategies was with 94.9% also higher than with other studies using NeVaTM [15,16,17], or other stent retrievers, for, e.g., of 80.3% in the Trevo (Stryker, Kalamazoo, USA), Stent Retriever Acute Stroke (TRACK) multicenter registry [29], of 85.3% for the Aperio stent retriever (Acandis, Pforzheim, Germany) [30], or 88% for the Solitaire (Medtronic, Irvine, USA) stent retriever in the SWIFT Prime study [4]. However, a recent study on NeVaTM stent retriever first-line use reported a final mTICI ≥2b of 94.2% similar to our data [18]. Importantly, our data shows that a considerably high amount of patients (86.7%) improved in final recanalization up to a mTICI ≥2b when NeVaTM stent retriever was used as second-line stent retriever again showing the potential of this device also as rescue strategy. Additionally, it has been shown that larger stent retrievers may improve first-pass rate and final mTICI score [11, 12]. In this context, the 4 × 30 mm NeVaTM stent retriever evaluated here is a rather small device. Importantly, NeVaTM devices are available up to 4.5 × 44 mm with 5 drop zones potentially even increasing recanalization rates (but maybe also potential complications), which will have to be evaluated in future studies.
Regarding the safety profile, our study did not find any device-related procedural complications, similarly to previous studies reporting adverse events only very rarely [15,16,17,18]. A previous study reported a high rate (48.3%) of vasospasms in the recanalized segment after thrombectomy with NeVaTM stent retriever [16]. Other studies on the NeVaTM device did not observe or explicitly report on such a high rate of vasospasm [15, 17, 18]. Our data observed intracranial vasospasms only in 2.6% which is similar to the rate reported in most studies with other thrombectomy devices [27, 31]. This might be explained by the difference in procedural technique, since Borggrefe et al. report to have only temporarily connected the catheter to a continuous flush line charged with nimodipine in case of observed vasospasms [16], while such nimodipine charged flush line is always connected to the guiding catheter at our center potentially preventing from such a high rate of periprocedural vasospasms. Meanwhile, we did observe a comparably high rate of intracranial hemorrhages at 24h after MT (33.3%). However, none of these was symptomatic confirming the low rate of SICH after usage of the NeVaTM stent retriever observed in previous studies [15,16,17]. Thus, NeVaTM stent retriever proofed as safe device for MT in our study cohort. However, one has to consider the high mechanical traction forces of this device implying the risk for complications such as caroticocavernous fistulas as reported elsewhere [16].
The above-mentioned recanalization rates resulted in a mean ± SD mRS of 3.3±2.0 and a rate of 35.9% with mRS 0–2 at discharge which is, considering the relatively small study cohort, comparable to a previous report (4.0±1.7 or 24%, respectively) [16] but lower than commonly reported after 90 days [15].
Limitations
This study is limited by its retrospective, single-center, and self-reported design and the relatively small number of patients included. Further, the study cohort has some heterogeneity regarding first- or second-line usage of the NeVaTM stent retriever, usage of balloon-guided or intermediate catheters, or variety in rescue strategies. Further, due to the lack of evidence-based recommendations, the use of the NeVaTM stent retriever as first- or second-line device was left to the discretion of the performing neurointerventionalist, thus leading to a potential selection bias. However, with this design, the study cohort represents a “real-world” dataset for the applicability and performance of the tested stent retriever. Further, this study does not include long-term clinical follow-up as the primary criteria were technical efficacy and safety profile. Future prospective and comparative studies in larger cohorts are needed to further characterize the optimal setting and limitations when to use the NeVaTM stent retriever as well as to compare long-term clinical outcome with those of other devices.
In conclusion, this study shows a high technical effectiveness and good safety profile of the NeVaTM stent retriever as first- and second-line device for mechanical thrombectomy in acute ischemic stroke.
References
Campbell BC et al (2015) Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 372:1009–1018. https://doi.org/10.1056/NEJMoa1414792
Goyal M et al (2015) Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372:1019–1030. https://doi.org/10.1056/NEJMoa1414905
Powers WJ et al (2019) Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50:e344–e418. https://doi.org/10.1161/STR.0000000000000211
Saver JL et al (2015) Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 372:2285–2295. https://doi.org/10.1056/NEJMoa1415061
Turc G et al (2019) European Stroke Organisation (ESO)- European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg 11:535–538. https://doi.org/10.1136/neurintsurg-2018-014568
Goyal M et al (2016) Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387:1723–1731. https://doi.org/10.1016/S0140-6736(16)00163-X
Goyal N et al (2019) Comparative safety and efficacy of modified TICI 2b and TICI 3 reperfusion in acute ischemic strokes treated with mechanical thrombectomy. Neurosurgery 84:680–686. https://doi.org/10.1093/neuros/nyy097
Jadhav AP et al (2022) First pass effect with neurothrombectomy for acute ischemic stroke: analysis of the systematic evaluation of patients treated with stroke devices for acute ischemic stroke registry. Stroke 53:e30–e32. https://doi.org/10.1161/STROKEAHA.121.035457
Zaidat OO et al (2018) First pass effect: a new measure for stroke thrombectomy devices. Stroke 49:660–666. https://doi.org/10.1161/STROKEAHA.117.020315
Zaidat OO et al (2023) MASTRO I: meta-analysis and systematic review of thrombectomy stent retriever outcomes: comparing functional, safety and recanalization outcomes between EmboTrap, Solitaire and Trevo in acute ischemic stroke. J Comp Eff Res 12:e230001. https://doi.org/10.57264/cer-2023-0001
Serna Candel C, Aguilar Perez M, Bazner H, Henkes H, Hellstern V (2021) First-pass reperfusion by mechanical thrombectomy in acute M1 occlusion: the size of retriever matters. Front Neurol 12:679402. https://doi.org/10.3389/fneur.2021.679402
Zaidat OO et al (2019) Impact of stent retriever size on clinical and angiographic outcomes in the STRATIS stroke thrombectomy registry. Stroke 50:441–447. https://doi.org/10.1161/STROKEAHA.118.022987
Machi P et al (2019) Experimental evaluation of the NeVa thrombectomy device a novel stent retriever conceived to improve efficacy of organized clot removal. J Neuroradiol 46:163–167. https://doi.org/10.1016/j.neurad.2018.03.005
Ulm AJ, Khachatryan T, Grigorian A, Nogueira RG (2018) Preclinical evaluation of the NeVaTM stent retriever: safety and efficacy in the swine thrombectomy model. Interv Neurol 7:205–217. https://doi.org/10.1159/000486288
Akpinar CK et al (2021) Favorable first-pass recanalization rates with NeVa thrombectomy device in acute stroke patients: initial clinical experience. Interv Neuroradiol 27:107–113. https://doi.org/10.1177/1591019920938223
Borggrefe J et al (2021) Mechanical thrombectomy with the novel NeVa M1 stent retriever: do the drop zones represent a risk or a benefit? World Neurosurg 148:e121–e129. https://doi.org/10.1016/j.wneu.2020.12.075
Ribo M et al (2020) Mechanical thrombectomy with a novel stent retriever with multifunctional zones: initial clinical experience with the NeVa thrombectomy device. J Neuroradiol 47:301–305. https://doi.org/10.1016/j.neurad.2019.03.007
Bajrami A et al (2022) First pass results of mechanical thrombectomy with two-drop zone NeVa(TM) device. Interv Neuroradiol 15910199221135309. https://doi.org/10.1177/15910199221135309
Yoo AJ et al (2023) Abstract WMP87: primary results from the CLEAR study of the safety and effectiveness of the neva stent retriever for large vessel thrombectomy. Stroke 54:AWMP87. https://doi.org/10.1161/str.54.suppl_1.WMP87
Ringleb P, Köhrmann M, Jansen O (2022) Akuttherapie des ischämischen Schlaganfalls, S2e-Leitlinie. Leitlinien für Diagnostik und Therapie in der Neurologie
Fugate JE, Klunder AM, Kallmes DF (2013) What is meant by "TICI"? AJNR Am J Neuroradiol 34:1792–1797. https://doi.org/10.3174/ajnr.A3496
Hacke W et al (2008) Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359:1317–1329. https://doi.org/10.1056/NEJMoa0804656
Bourcier R et al (2019) More than three passes of stent retriever is an independent predictor of parenchymal hematoma in acute ischemic stroke. J Neurointerv Surg 11:625–629. https://doi.org/10.1136/neurintsurg-2018-014380
Garcia-Tornel A et al (2019) When to stop. Stroke 50:1781–1788. https://doi.org/10.1161/STROKEAHA.119.025088
Garcia-Tornel A et al (2020) Sudden recanalization: a game-changing factor in endovascular treatment of large vessel occlusion strokes. Stroke 51:1313–1316. https://doi.org/10.1161/STROKEAHA.119.028787
Jindal G et al (2019) Beyond the first pass: revascularization remains critical in stroke thrombectomy. J Neurointerv Surg 11:1095–1099. https://doi.org/10.1136/neurintsurg-2019-014773
Turk AS 3rd et al (2019) Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet 393:998–1008. https://doi.org/10.1016/S0140-6736(19)30297-1
Zaidat OO et al (2018) Primary results of the multicenter ARISE II study (analysis of revascularization in ischemic stroke with EmboTrap). Stroke 49:1107–1115. https://doi.org/10.1161/STROKEAHA.117.020125
Zaidat OO et al (2018) TREVO stent-retriever mechanical thrombectomy for acute ischemic stroke secondary to large vessel occlusion registry. J Neurointerv Surg 10:516–524. https://doi.org/10.1136/neurintsurg-2017-013328
Kaschner MG et al (2019) One-year single-center experience with the Aperio thrombectomy device in large vessel occlusion in the anterior circulation: safety, efficacy, and clinical outcome. Neurol Sci 40:1443–1451. https://doi.org/10.1007/s10072-019-03861-z
Lapergue B et al (2017) Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER randomized clinical trial. JAMA 318:443–452. https://doi.org/10.1001/jama.2017.9644
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Ethical approval
The study was approved by the local ethics committee (ID: 2022-243-f-S).
Informed consent
Informed patient consent was waived due to the retrospective character of this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Masthoff, M., Krähling, H., Akkurt, B.H. et al. Evaluation of effectiveness and safety of the multizone NeVaTM stent retriever for mechanical thrombectomy in ischemic stroke. Neuroradiology 65, 1777–1785 (2023). https://doi.org/10.1007/s00234-023-03236-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-023-03236-4