Abstract
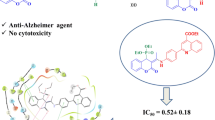
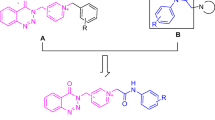
Rivastigmine has been prescribed for the therapy of Alzheimer’s disease (AD) symptoms. This drug is classified in the carbamate derivative group that has dual activity against acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). According to the structure of Rivastigmine and its performance, a new series of 5-aryl-1,3,4-oxadiazole-2-carbamothioate compounds I–XI was synthesized using structure-based drug discovery approaches. For this purpose a set of these compounds were designed with computational docking method and their interactions with amino acid residues in the active sites of AChE and BuChE checked out. The structures of synthesized compounds were established by physicochemical and spectroscopic methods. The carbamoyl moiety of Rivastigmine structure was modified to carbamothioate and the effects of 1,3,4-oxadiazole heterocycle as a pharmacophoric nucleus were investigated. The potential of the synthesized compounds I–XI was evaluated against two most known agents of AD (AChE and BuChE) to determine their IC50 values. The results of the docking showed the range of binding affinity for the best poses of ten individual conformers for any compounds (I–XI) was between −7.81 (VI) and −6.75 (II) kcal/mol. The results of biological experiments displayed that most synthetic compounds (I–VIII) showed moderate to excellent selective activity range against BuChE (0.51–69.44 µM). In vitro cytotoxicity evaluation of these compounds (I–XI) by MTT assay on human dermal fibroblast (HDF) cell line exhibited no activity against HDF. The compound VI [S-(5-(p-tolyl)-1,3,4-oxadiazol-2-yl) ethyl(methyl)carbamothioate] showed the most stable binding affinity (−7.81 kcal/mol) and the lowest IC50 value (0.51 µM) in comparison with Rivastigmine with 7.72 µM and Donepezil with 5.20 µM against BuChE.






Similar content being viewed by others
References
Abolhasani MH, Safavi M, Goodarzi MT, Kassaee SM, Azin M (2018) Identification and anti-cancer activity in 2D and 3D cell culture evaluation of an Iranian isolated marine microalgae Picochlorum sp. RCC486. Daru J Pharm Sci 26:105–116
Bajda M, Łatka K, Hebda M, Jonczyk J, Malawska B (2018) Novel carbamate derivatives as selective butyrylcholinesterase inhibitors. Bioorg Chem 78:29–38
Bingul M, Saglam MF, Kandemir H, Boga M, Sengul IF (2019) Synthesis of indole-2-carbohydrazides and 2-(indol-2-yl)-1,3,4-oxadiazoles as antioxidants and their acetylcholinesterase inhibition properties. Monatsh Chem https://doi.org/10.1007/s00706-019-02462-y
Boström J, Hogner A, Llinas A, Wellner E, Plowright AT (2012) Oxadiazoles in medicinal chemistry. J Med Chem 55:1817–1830
Danish M, Raza MA, Anwar U, Rashid U, Ahmed Z (2019) Differential functional theory and molecular docking studies of newly synthesized carbamates. J Chin Chem Soc https://doi.org/10.1002/jccs.201800068
Faraji L, Nadri H, Moradi A, Bukhari SN, Pakseresht B, Moghadam FH, Moghimi S, Abdollahi M, Khoobi M, Foroumadi A (2019) Aminoalkyl-substituted flavonoids: synthesis, cholinesterase inhibition, β-amyloid aggregation, and neuroprotective study. Med Chem Res 28:974–983
Ghobadian R, Esfandyari R, Nadri H, Moradi A, Mahdavi M, Akbarzadeh T, Khaleghzadeh-Ahangar H, Edraki N, Sharifzadeh M, Amini M (2019) Design, synthesis, in vivo and in vitro studies of 1,2,3,4-tetrahydro-9H-carbazole derivatives, highly selective and potent butyrylcholinesterase inhibitors. Mol Divers https://doi.org/10.1007/s11030-019-09943-6
Gudi Y, Mangali MS, Gundala S, Venkatapuram P, Adivireddy P (2018) Synthesis, characterization, and bioassay of a new class of pyrazolyl/isoxazolyl oxadiazoles. Monatsh Chem 149:2311–2326
Hariri R, Afshar Z, Mahdavi M, Safavi M, Saeedi M, Najafi Z, Sabourian R, Karimpour-Razkenari E, Edraki N, Moghadam FH, Shafiee A, Khanavi M, Akbarzadeh T (2016) Novel tacrine-based pyrano[3’,4’:5,6]pyrano[2,3-b]quinolinones: synthesis and cholinesterase inhibitory activity. Arch Pharm Chem Life Sci 349:1–10
Kang L, Gao XH, Liu HR, Men X, Wu HN, Cui PW, Oldfield E, Yan JY (2018) Structure–activity relationship investigation of coumarin–chalcone hybrids with diverse side-chains as acetylcholinesterase and butyrylcholinesterase inhibitors. Mol Divers 22:896–906
Karabanovich G, Zemanová J, Smutný T, Székely R, Šarkan M, Centárová I, Vocat A, Pávková I, Čonka P, Němeček J, Stolaříková J, Vejsová M, Vávrová K, Klimešová V, Hrabálek A, Pávek P, Cole ST, Mikušová K, Roh J (2016) Development of 3,5-dinitrobenzylsulfanyl-1,3,4-oxadiazoles and thiadiazoles as selective antitubercular agents active against replicating and nonreplicating mycobacterium tuberculosis. J Med Chem 59:2362–2380
Koola MM, Praharaj SK, Pillai A (2019) Galantamine-memantine combination as an antioxidant treatment for schizophrenia. Curr Behav Neurosci Rep. 6:37–50
Li P, Shi L, Gao MN, Yang X, Xue W, Jin LH, Hu DY, Song BA (2015) Antibacterial activities against rice bacterial leaf blight and tomato bacterial wilt of 2-mercapto-5-substituted-1,3,4-oxadiazole/thiadiazole derivatives. Bioorg Med Chem Lett 25:481–484
Mahajan PG, Dige NC, Vanjare BD, Raza H, Hassan M, Seo SY, Kim CH, Lee KH (2019) Synthesis and biological evaluation of 1,2,4-triazolidine-3-thiones as potent acetylcholinesterase inhibitors: in vitro and in silico analysis through kinetics, chemoinformatics and computational approaches. Mol Divers https://doi.org/10.1007/s11030-019-09983-y
Makhaeva GF, Kovaleva VN, Lushchekina SV, Rudakova EV, Boltneva NP, Proshin AN, Lednev BV, Serkov IV, Bachurin SO (2018) Conjugates of Tacrine and its cyclic homologues with p toluenesulfonamide as novel acetylcholinesterase and butyrylcholinesterase inhibitors. Dokl Biochem Biophys 483:369–373
Mohammadi-Khanaposhtani M, Mahdavi M, Saeedi M, Sabourian R, Safavi M, Khanavi M, Foroumadi A, Shafiee A, Akbarzadeh T (2015) Design, synthesis, biological evaluation, and docking study of acetylcholinesterase inhibitors: new acridone-1,2,4-oxadiazole-1,2,3-triazole hybrids. Chem Biol Drug Des 86:1425–1432
Morán-Díaz JR, Jiménez-Vázquez HA, Gómez-Pliego R, Arellano-Mendoza MG, Quintana-Zavala D, Guevara-Salazar JA (2019) Correlation study of antibacterial activity and spectrum of Penicillins through a structure-activity relationship analysis. Med Chem Res 28:1529–1546
Najafi Z, Mahdavi M, Saeedi M, Sabouriane R, Khanavi M, Safavi M, Tehrani MB, Shafieeh A, Foroumadih A, Akbarzadeh T (2016) 1,2,3-Triazole-Isoxazole based acetylcholinesterase inhibitors: synthesis, biological evaluation and docking study. Lett Drug Des Disco 14:58–65
Nayak SG, Poojary B (2019) A review on the preparation of 1,3,4-oxadiazoles from the dehydration of hydrazines and study of their biological roles. Chem Afr https://doi.org/10.1007/s42250-019-00084-9
Nunes N, Rosa GP, Ferraz1 S, Barreto MC, Carvalho MP (2019) Fatty acid composition, TLC screening, ATR-FTIR analysis, anti-cholinesterase activity, and in vitro cytotoxicity to A549 tumor cell line of extracts of 3 macroalgae collected in Madeira. J Appl Phycol https://doi.org/10.1007/s10811-019-01884-9
Özdemir Z, Yılmaz H, Sar S, Karakurt A, Şeno FS, Uysa M (2017) Design, synthesis, and molecular modeling of new 3(2H)-pyridazinone derivatives as acetylcholinesterase/butyrylcholinesterase inhibitors. Med Chem Res 26:2293–2308
Pordeli M, Nakhjiri M, Safavi M, Ardestani SK, Foroumadi A (2017) Anticancer effects of synthetic hexahydrobenzo [g]chromen-4-one derivatives on human breast cancer cell lines. Breast Cancer 24:299–311
Purgatorio R, Candia MD, Catto M, Carrieri A, Pisani L, Palma AD, Toma M, Ivanova OA, Voskressensky LG, Altomare CD (2019) Investigating 1,2,3,4,5,6-hexahydroazepino[4,3-b]indole as scaffold of butyrylcholinesterase-selective inhibitors with additional neuroprotective activities for Alzheimer's disease. Eur J Med Chem 177:414–424
Rohand T, Ramli Y, Baruah M, Budka J, Das AM (2019) Synthesis, structure elucidation and antimicrobial properties of new bis-1,3,4-oxadiazole derivatives. Pharm Chem J 53:150–154
Roy PP, Banjare P, Verma S, Singh J (2019) Acute rat and mouse oral toxicity determination of anticholinesterase inhibitor carbamate pesticides: a QSTR approach. Mol Inform 38:1800151–1800167
Saeed A, Shah MS, Larik FA, Khan SU, Channar PA, Flörke U, Iqbal J (2017) Synthesis, computational studies and biological evaluation of new 1-acetyl-3-aryl thiourea derivatives as potent cholinesterase inhibitors. Med Chem Res 26:1635–1646
Saeedi M, Safavi M, Karimpour-Razkenari E, Mahdavi M, Edraki N, Moghadam FH, Khanavi M, Akbarzadeh T (2017) Synthesis of novel chromenones linked to 1,2,3-triazole ring system: Investigation of biological activities against Alzheimer’s disease. Bioorg Chem 70:86–93
San Juan AA, Bacalhau P, Goth A, Caldeira AT, Martins R, Burke AJ (2016) Insights into (S)-Rivastigmine inhibition of butyrylcholinesterase (BuChE): molecular docking and saturation transfer difference NMR (STD-NMR). Bioorg Chem 67:105–109
Sekhar MM, Yamini G, Divya KR, Padmavathi V, Padmaja A (2019) Synthesis and bioassay of a new class of disubstituted 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazoles. Med Chem Res 28:1049–1062
Shamsimeymandi R, Pourshojaei Y, Eskandari K, Mohammadi-Khanaposhtani M, Abiri A, Khodadadi A, Langarizadeh A, Sharififar F, Amirheidari B, Akbarzadeh T, Lotfian H, Foroumadi A, Asadipour A (2019) Design, synthesis, biological evaluation, and molecular dynamics of novel cholinesterase inhibitors as anti-Alzheimer's agents. Arch Pharm Chem Life Sci 352:1800352–1800363
Shi DH, Ma XD, Liu YW, Min W, Yin FJ, Tang ZM, Song MQ, Lu C, Song XK, Liu WW, Dong T (2018) Synthesis, crystal structure and biological evaluation of novel 2-phenylthiazole derivatives as butyrylcholinesterase inhibitors. J Chem Res 42:366–370
Shingare RM, Patil YS, Sangshetti JN, Patil RB, Rajani DP, Madje BR (2018) Synthesis, biological evaluation and docking study of some novel isoxazole clubbed 1,3,4-oxadiazoles derivatives. Med Chem Res 27:1283–1291
Simurova NV, Maiboroda OI (2019) Synthesis of mono- and disubstituted 1,3,4-oxadiazoles (microreview). Chem Heterocycl Compd 55:604–606
Turkan F, Cetin A, Taslimi P, Karaman HS, Gulçin İ (2019) Synthesis, characterization, molecular docking and biological activities of novel pyrazoline derivatives. Arch Pharm Chem Life Sci 352:1800359–1800370
Yadav E, Singh D, Debnath B, Rathee P, Yadav P, Verma A (2019) Molecular docking and cognitive impairment attenuating efect of phenolic compound rich fraction of Trianthema portulacastrum in scopolamine induced Alzheimer’s disease like condition. Neurochem Res 44:1665–1677
Yan L, Deng M, Chen A, Li Y, Zhang W, Du ZY, Dong CZ, Meunier B, Chen H (2019) Synthesis of N-pyrimidin[1,3,4]oxadiazoles and N-pyrimidin[1,3,4]-thiadiazoles from 1,3,4-oxadiazol-2-amines and 1,3,4-thiadiazol-2-amines via Pd-catalyzed heteroarylamination. Tetrahedron Lett 60:1359–1362
Yazdani M, Edraki N, Badri R, Khoshneviszadeh M, Iraji A, Firuzi O (2019) 5,6-Diphenyl triazine-thio methyl triazole hybrid as a new Alzheimer’s disease modifying agents. Mol Divers https://doi.org/10.1007/s11030-019-09970-3
Yusufzai SK, Khan MS, Sulaiman O, Osman H, Lamjin DN (2018) Molecular docking studies of coumarin hybrids as potential acetylcholinesterase, butyrylcholinesterase, monoamine oxidase A/B and β-amyloid inhibitors for Alzheimer’s disease. Chem Cent J 12:128–185
Zengin M, Unsal-Tan O, Küçükkýlýnç TT, Ayazgok B, Balkan A (2019) Design and synthesis of 2-substituted phenyl benzo[d]thiazole derivatives and their b-amyloid aggregation and cholinesterase inhibitory activities. Pharm Chem J 53:322–328
Zhang XZ, Xu Y, Jian MM, Yang K, Ma ZY (2019) Synthesis, in vitro assays, molecular docking, theoretical ADMET prediction, and evaluation of 4 methoxy-phenylthiazole-2-amine derivatives as acetylcholinesterase inhibitors. Med Chem Res 28:1683–1693
Acknowledgements
The authors are thankful to Department of Chemical Technologies, Iranian Research Organization for Science and Technology (IROST) for financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fallah, A., Mohanazadeh, F. & Safavi, M. Design, synthesis, and in vitro evaluation of novel 1,3,4-oxadiazolecarbamothioate derivatives of Rivastigmine as selective inhibitors of BuChE. Med Chem Res 29, 341–355 (2020). https://doi.org/10.1007/s00044-019-02475-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02475-6